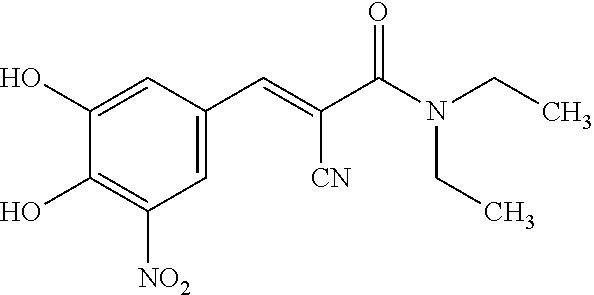
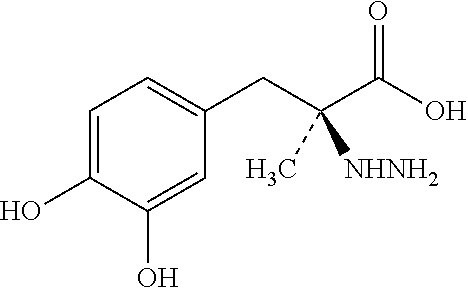
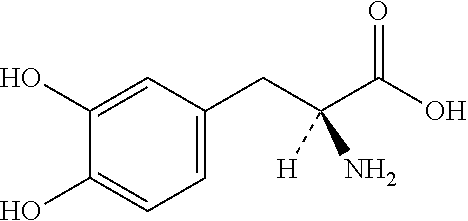
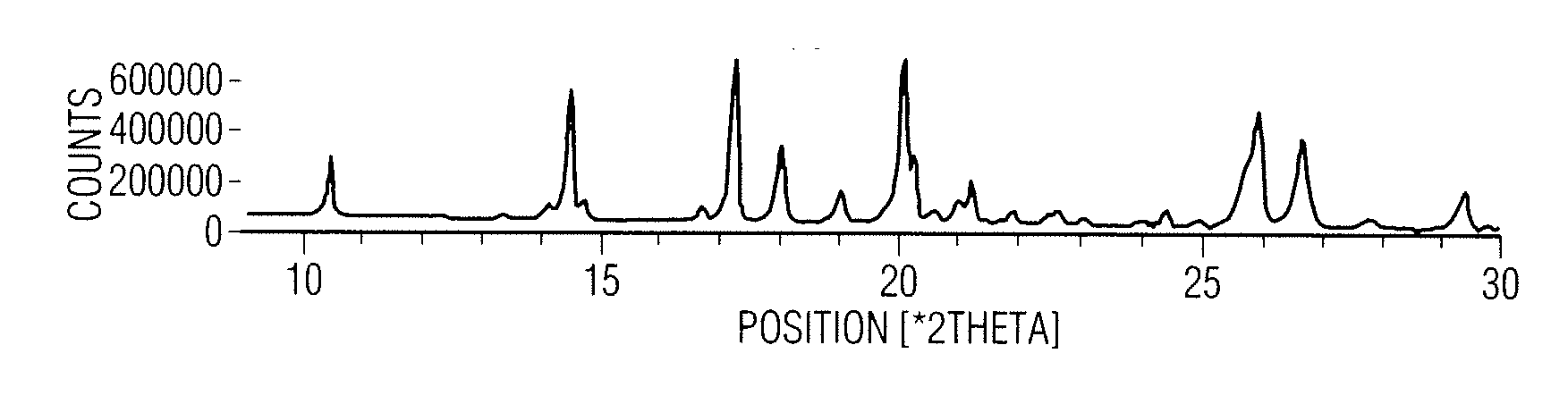
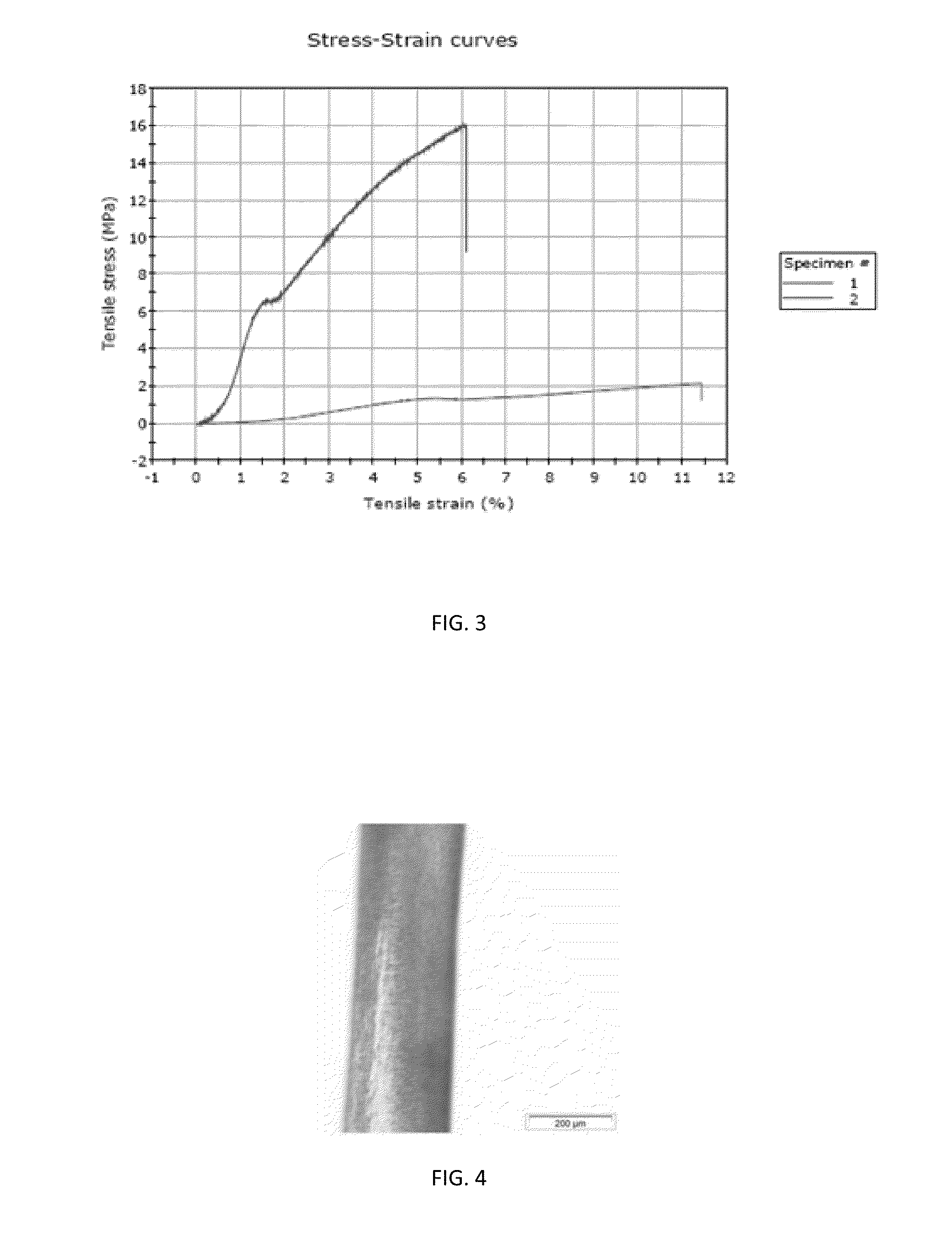
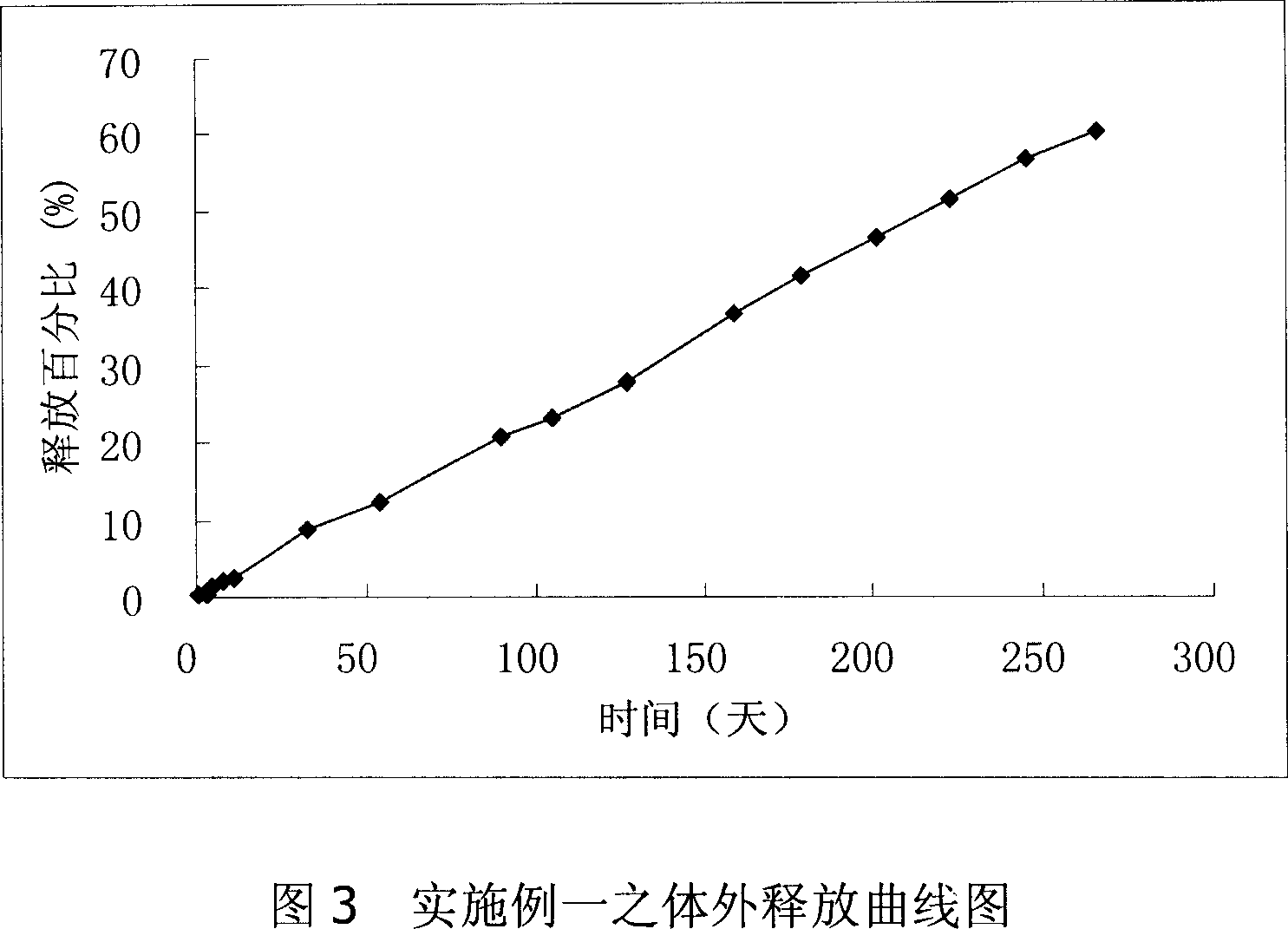
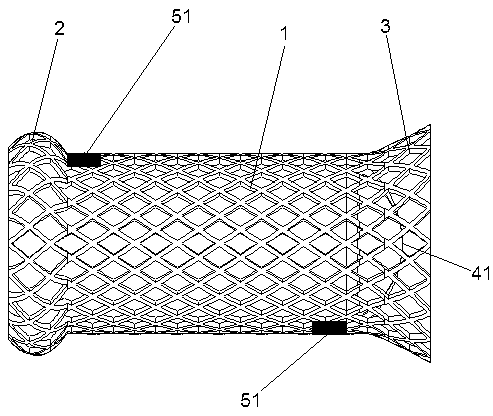
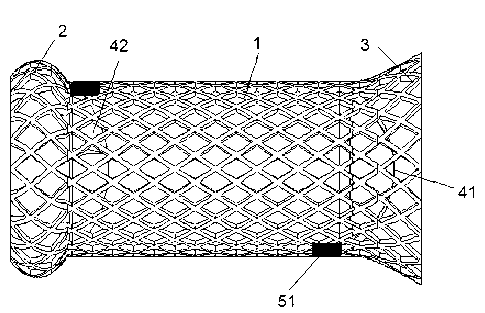
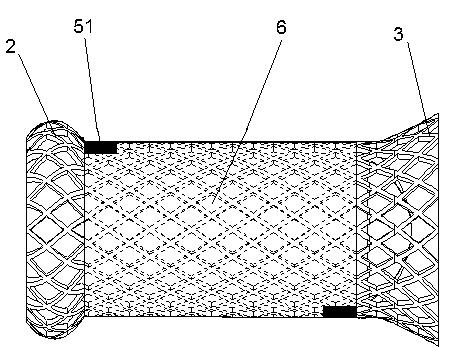
Patents
Literature
394 results about "Slow release drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage forms of a drug that act over a period of time by controlled-release processes or technology.
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
InactiveUS7157099B2Easy to prepareOrganic active ingredientsPeptide/protein ingredientsParenteral nutritionBULK ACTIVE INGREDIENT
Owner:ITALFARMACO SPA
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Stent coated with a sustained-release drug delivery and method for use thereof
ActiveUS7279175B2Reduce deliveryReduce solubilitySuture equipmentsAntibacterial agentsSustained release drugDrug compound
An intraluminal medical device comprises a stent having a coating applied to at least part of an interior surface, an exterior surface, or both. The coating comprises a sustained release formulation of a combination of pharmaceutical compounds dispersed within a biologically tolerated polymer composition. The choice of the combination of pharmaceutical compounds are intended to reduce neointimal hyperplasia restenosis.
Owner:PSIVIDA US INC
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Modulation of release from dry powder formulations
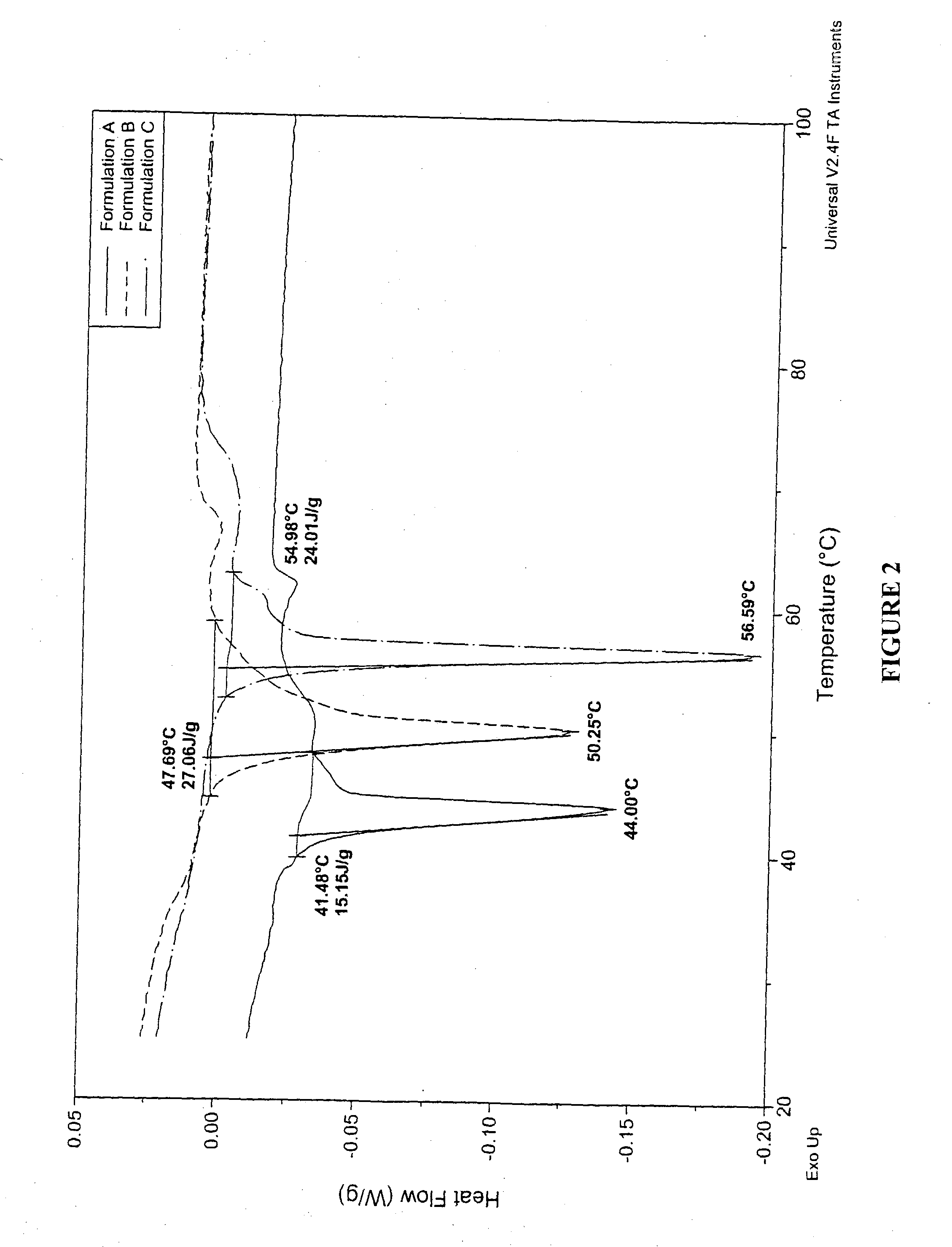
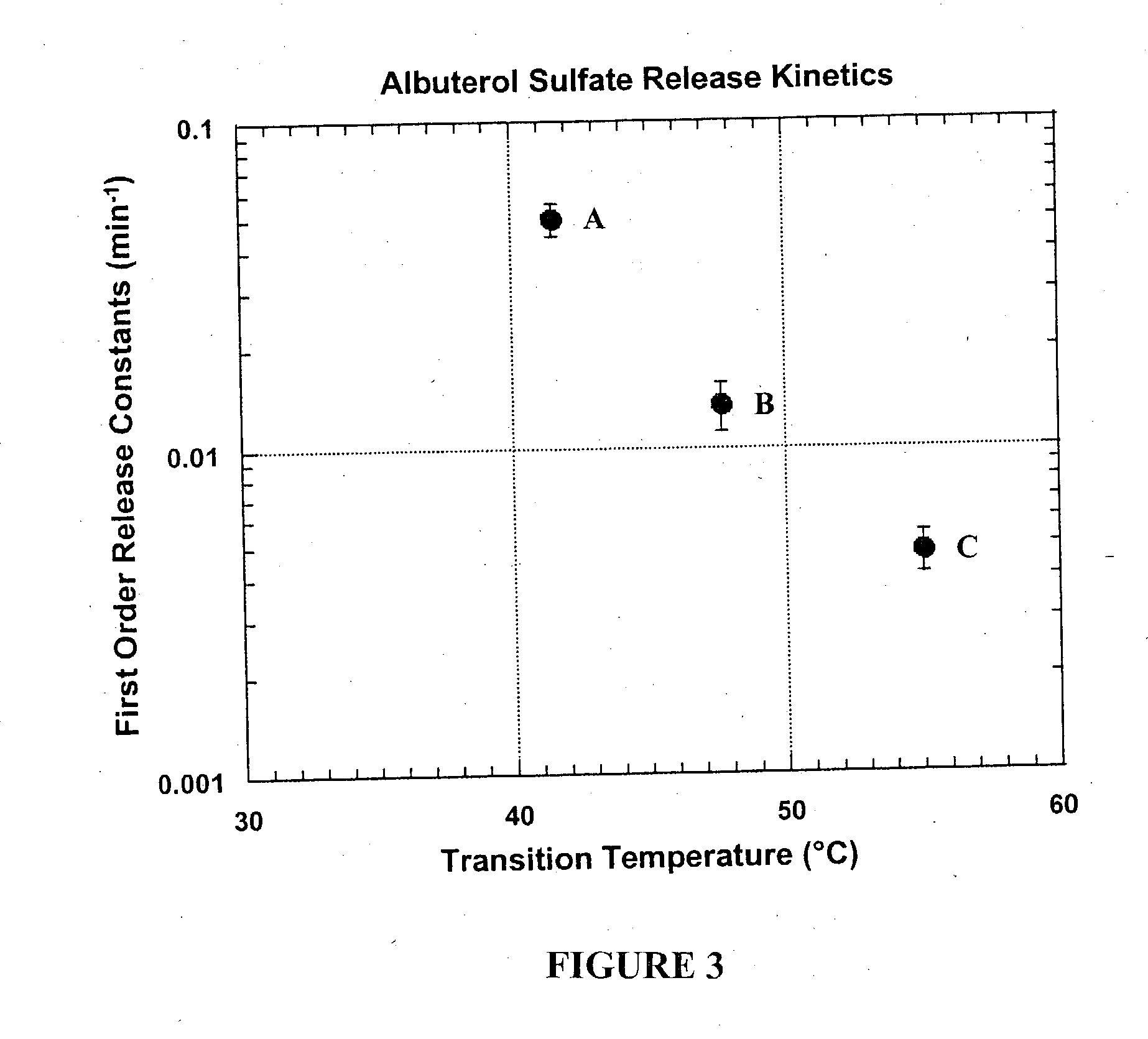
InactiveUS20050003003A1Raise the transition temperaturePowder deliveryOrganic active ingredientsMedicinePharmacologic action
Particles which include a bioactive agent are prepared to have a desired matrix transition temperature. Delivery of the particles via the pulmonary system results in modulation of drug release from the particles. Sustained release and / or sustained pharmacologic action of the drug can be obtained by forming particles which include a combination of phospholipids that are miscible in one another and have a high matrix transition temperature.
Owner:CIVITAS THERAPEUTICS
Treatment of ocular disease
A formulation to treat ocular conditions such as dry eye disease, as well as other conditions, is disclosed. Rapamycin and / or ascomycin is administered intraocularly, such as by topical application, injection into the eye, or implantation in or on the eye. For example, a topical administration may contain between about 50 pg / ml drug to about 50 μg / ml drug in a formulation which may be applied at bedtime or throughout the day. For injection, a dose of about 50 pg / ml to about 200 μg / ml may be used. Rapamycin and / or ascomycin may also be administered in milligram quantities as a surgical implant, for example, in a diffusible walled reservoir sutured to the wall of the sclera, or may be contained within an inert carrier such as microspheres or liposomes to provide a slow-release drug delivery system.
Owner:PEYMAN GHOLAM A DR
Treatment of ocular disease
InactiveUS20050025810A1Prevent and decrease time of onsetReduce severityBiocideSenses disorderMicrosphereSurgical implant
A formulation to treat ocular conditions such as dry eye disease, as well as other conditions, is disclosed. Rapamycin and / or ascomycin is administered intraocularly, such as by topical application, injection into the eye, or implantation in or on the eye. For example, a topical administration may contain between about 50 pg / ml drug to about 50 μg / ml drug in a formulation which may be applied at bedtime or throughout the day. For injection, a dose of about 50 pg / mi to about 200 μg / ml may be used. Rapamycin and / or ascomycin may also be administered in milligram quantities as a surgical implant, for example, in a diffusible walled reservoir sutured to the wall of the sclera, or may be contained within an inert carrier such as microspheres or liposomes to provide a slow-release drug delivery system.
Owner:PEYMAN GHOLAM A DR
Sustained-release drug delivery compositions and methods
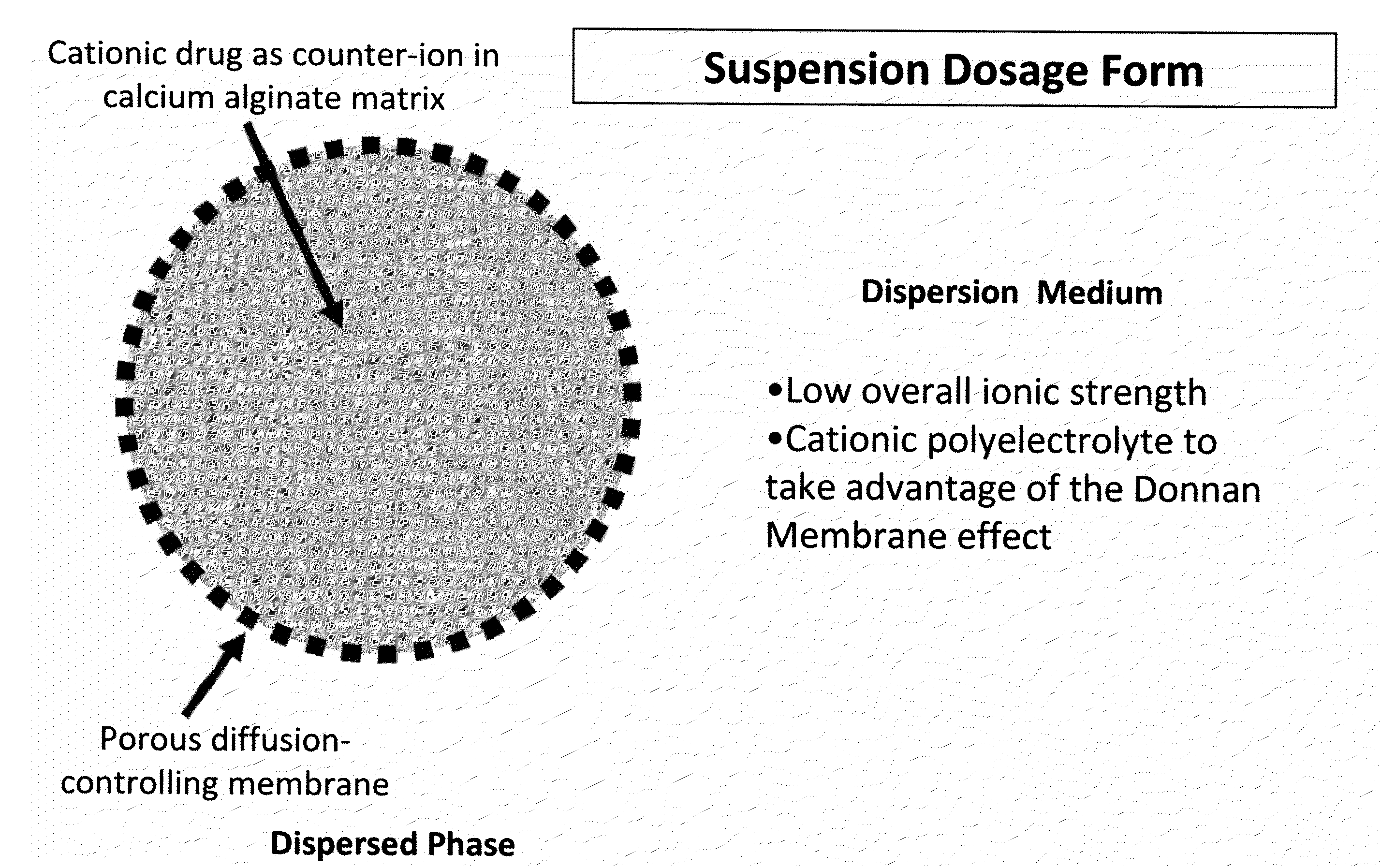
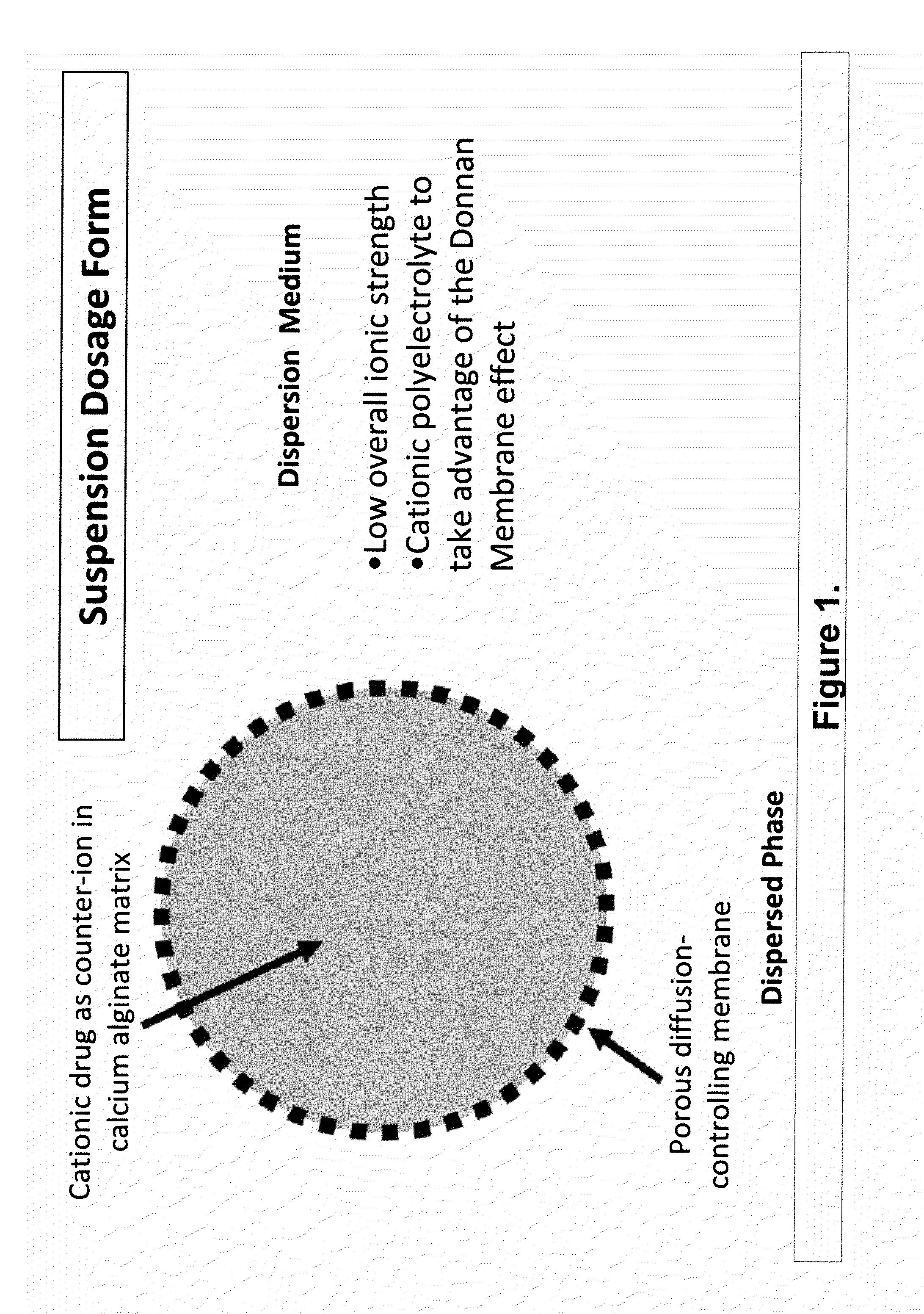
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
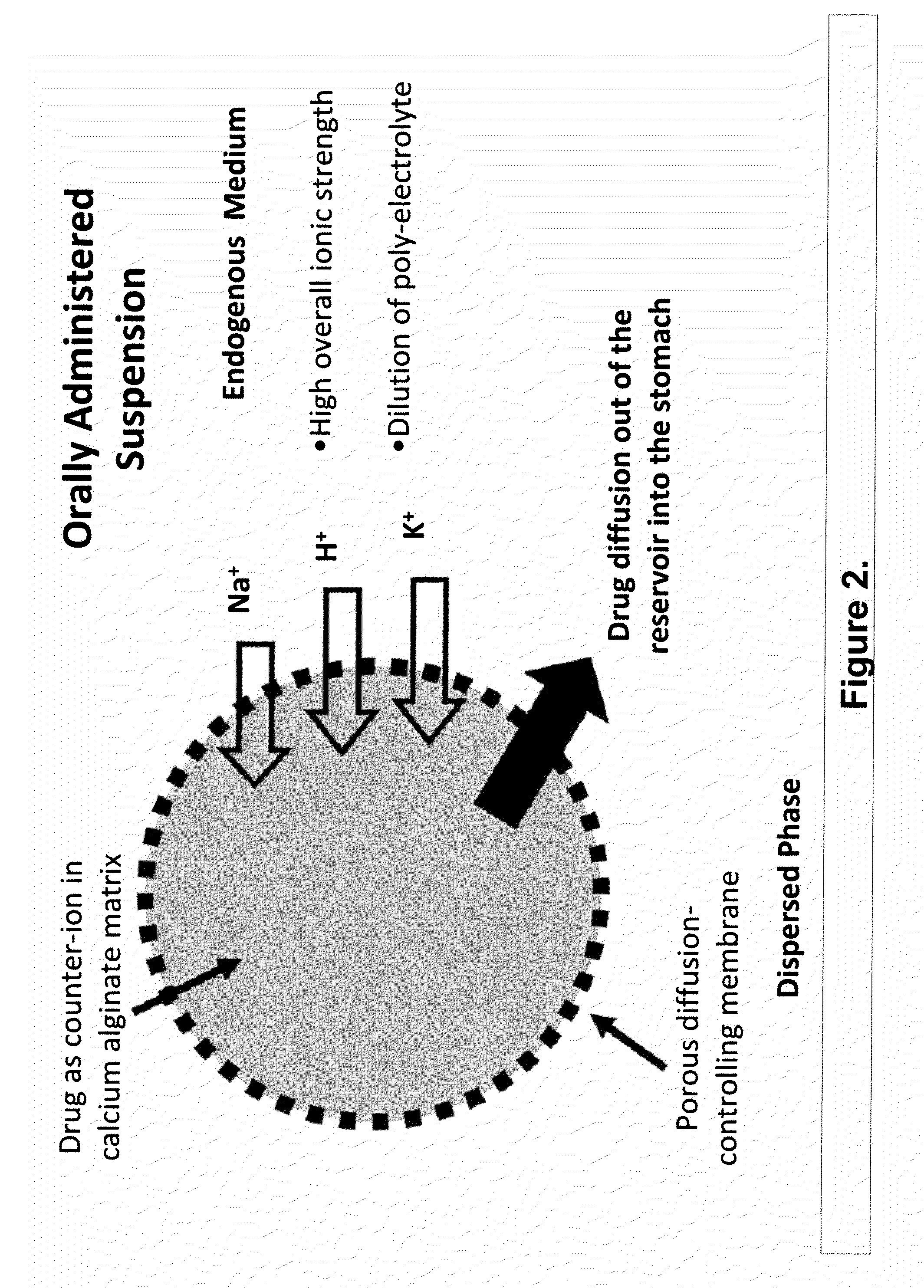
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
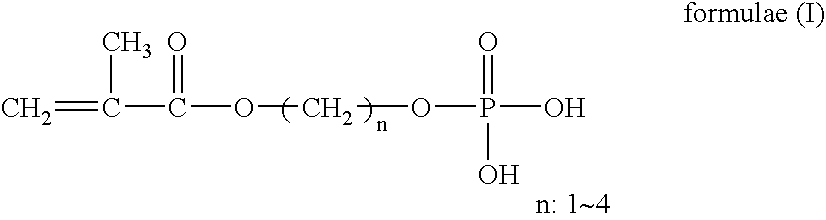
Ophthalmic lenses capable of sustained drug release and preservative solutions therefor
ActiveUS20060187410A1Prevent elutionStay in shapePowder deliveryEye treatmentHydrophilic monomerMethacrylate
An object of the present invention is to provide a practical ophthalmic lens which has an effect of effectively retaining and sustainedly releasing a drug and has form stability before and after release of the drug, wherein the ionic polymer gel having sustained drug releasability can regulate the amount of the drug included therein, depending on the efficacy of the drug used, and storing solution for a practical ophthalmic lens. The present invention relates to a drug delivery ophthalmic lens comprising a cationic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer having a hydroxyl group in its molecule, at least one member selected from specific phosphate group-containing methacrylates a monomer having a nitrogen atom in its side chain, and a monomer copolymerizable with these components, and also relates to a drug delivery ophthalmic lens comprising an anionic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer, cationic and anionic monomers, and a monomer copolymerizable with these components, wherein the copolymer contains the anionic monomer in a ratio of 30 to 90 mol % to the cationic monomer, and also relates to storing solution for a practical ophthalmic lens.
Owner:VIEWDLE INC
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
InactiveUS20110229561A1Reducing “ wearing off ” phenomenonReduce wearBiocideNervous disorderTriple combinationCarbidopa
There is provided an extended release pharmaceutical composition comprising from about 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also provides an extended release pharmaceutical composition comprising triple combination of from about 30 mg to about 300 mg of levodopa, 10 mg to about 100 mg of carbidopa and 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:WOCKHARDT LTD
Treatment of ocular disease
InactiveUS20060263409A1Reduce onset timeReduce severityBiocideSenses disorderMicrosphereSurgical implant
A formulation to treat ocular conditions such as dry eye disease, as well as other conditions, is disclosed. Rapamycin and / or ascomycin is administered intraocularly, such as by topical application, injection into the eye, or implantation in or on the eye. For example, a topical administration may contain between about 50 pg / ml drug to about 50 μg / ml drug in a formulation which may be applied at bedtime or throughout the day. For injection, a dose of about 50 pg / ml to about 200 μg / ml may be used. Rapamycin and / or ascomycin may also be administered in milligram quantities as a surgical implant, for example, in a diffusible walled reservoir sutured to the wall of the sclera, or may be contained within an inert carrier such as microspheres or liposomes to provide a slow-release drug delivery system.
Owner:MINU
Non-benzodiazepine hypnotic compositions
InactiveUS20070098788A1Quick releaseAct quicklyPowder deliveryOrganic active ingredientsImmediate releaseEnantiomer
Pharmaceutical compositions comprising non-benzodiazepine hypnotic drugs or their pharmaceutically acceptable salts, solvates, enantiomers or mixtures and processes for preparing the same. The invention also includes immediate release and extended release pharmaceutical compositions comprising non-benzodiazepine hypnotic drugs, useful for sleep induction and sleep maintenance.
Owner:DR REDDYS LAB LTD +1
Process for preparing novel medicine for breviscapine having function of promoting blood circulation and removing blood stasis
InactiveCN1385163AAvoid valley effectGuaranteed validityOrganic active ingredientsPill deliveryRemove bloodAnalgesic effect
The present invention discloses a slow-released medicine preparation with the function of promoting blood circulation, removing staiss, removing obstruction in the channels to relieve pain. In particular it is a slow-released medicine preparation containing breviscapine. The described breviscapine is scutellarin or 4-hydroxy ba icalein-7-O-beta-D-pyrangluconate methyl ester.
Owner:SICHUAN YIBIN WULIANGYE GROUP YIBIN PHARMA
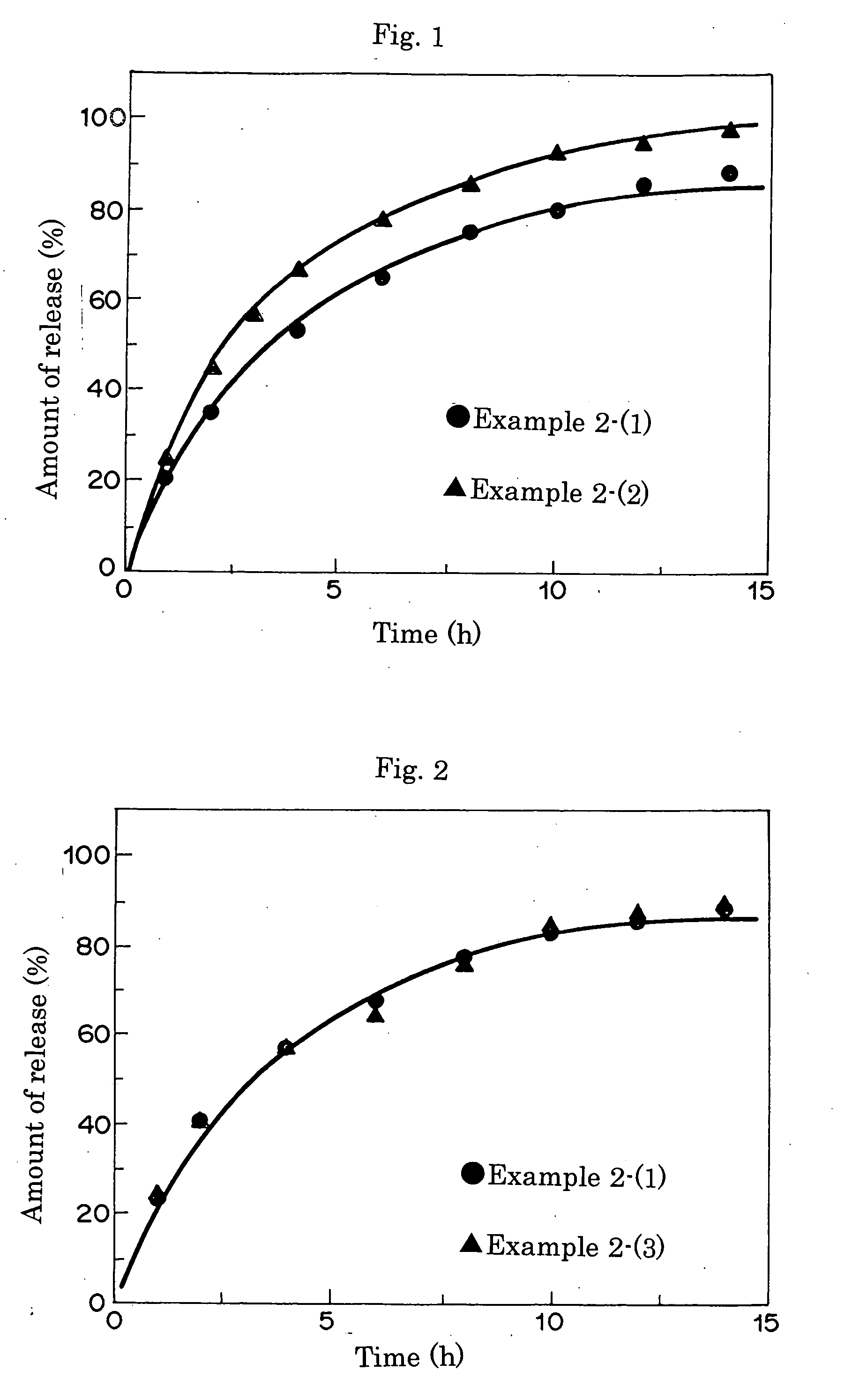
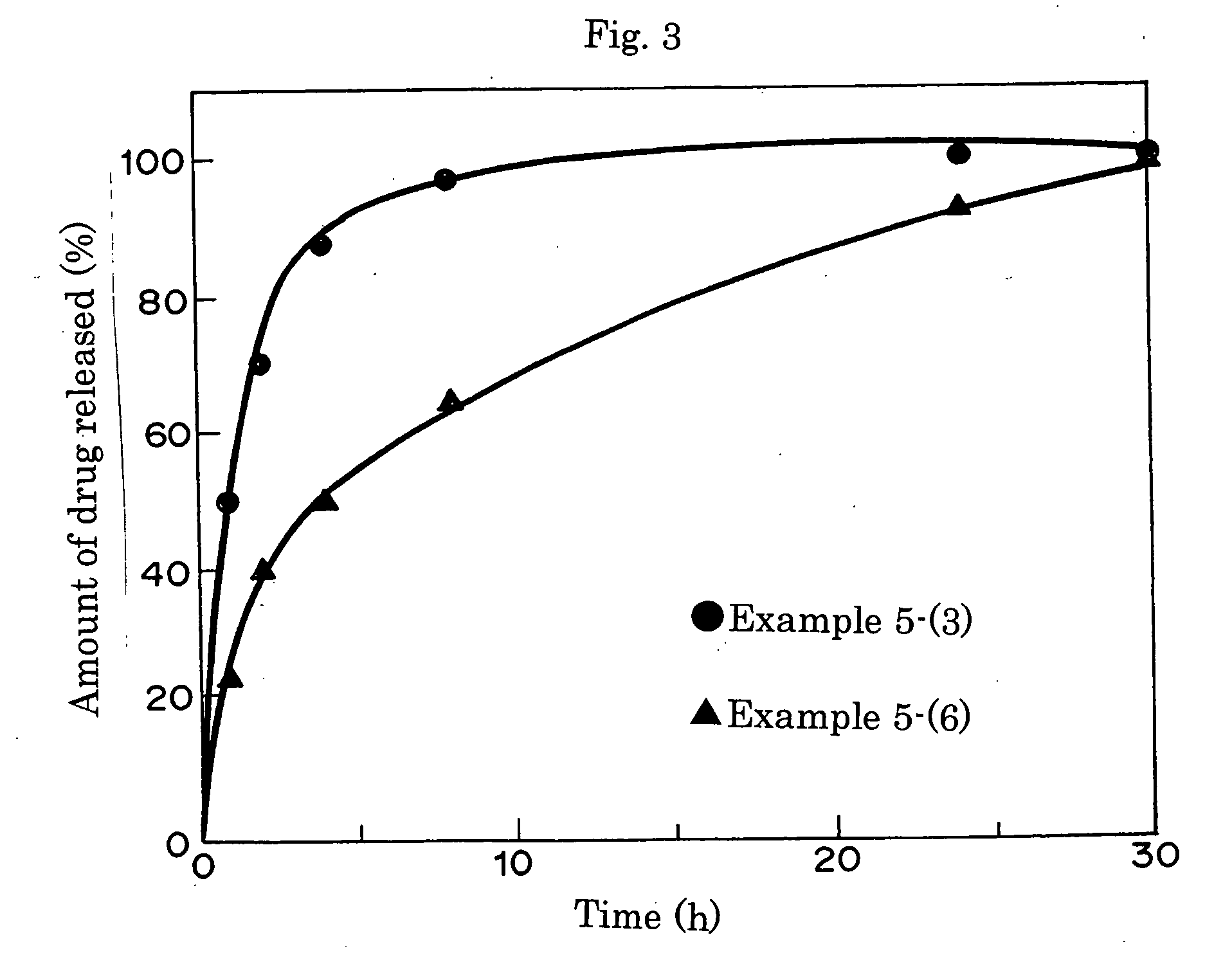
Pharmaceutical formulations for sustained release
InactiveUS20060293217A1Sustained deliveryOrganic active ingredientsBiocideHigh concentrationActive component
Sustained delivery pharmaceutical compositions comprising a solid ionic complex of a pharmaceutically active compound and an ionic macromolecule are provided by the present invention. The pharmaceutical compositions of the invention allow for loading of high concentrations of pharmaceutically active compounds and for delivery of a pharmaceutically active compound for prolonged periods of time, e.g., one month, after administration. Methods for preparing these pharmaceutical compositions, as well as methods of using them to treat a subject are also provided.
Owner:PRAECIS PHARM INC
Injectable Sustained-Release Pharmaceutical Formulation and the Preparation Method Thereof
InactiveUS20110091420A1Good effectGood sustained release effectBiocideCalcitoninsOrganic acidSolvent
Disclosed are an injectable sustained-release pharmaceutical formulation and a process for preparing the same. In some embodiments, the formulation comprises an active ingredient in a therapeutically effective amount, an amphipathic molecule, an organic acid and / or a salt thereof which is hardly soluble in water, and an oily solvent. The injectable sustained-release pharmaceutical formulation provides a good sustained-release effect for various active ingredients, in particular peptides, proteins, nucleic acids and saccharides.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A +1
Complete degradable absorbent medicine slow-release magnesium alloy bracket and use thereof
ActiveCN101385875AControlled bioabsorption and degradation propertiesHigh strengthStentsSurgeryBiological bodyVisibility
The invention relates to the technical field of medical apparatus, in particular to a drug-release magnesium alloy stent which can be completely degraded and adsorbed, with the period of drug release being about 30 days. The stent is obtained from a magnesium alloy precise tube that is cut through laser; the surface of the stent has a double-layer coating structure, with the inner layer being a temporarily protective layer of a magnesium alloy substrate and the outer layer a degradable polymer film which is mixed with drugs. In addition, the stent is visual in X-ray by means of marks at both sides or on the surface. The drug-release magnesium alloy stent is not only provided with good mechanical property, but also good visibility in X-ray, and can, by the option of slow-release drugs, reduce the restenosis occurrence after being implanted. In addition, the stent can be gradually degraded in and adsorbed by a biosome and used as a stent in a cardiovascular system or other cavities.
Owner:北京金信科创生物技术有限责任公司
Sustained release pharmaceutical compositions
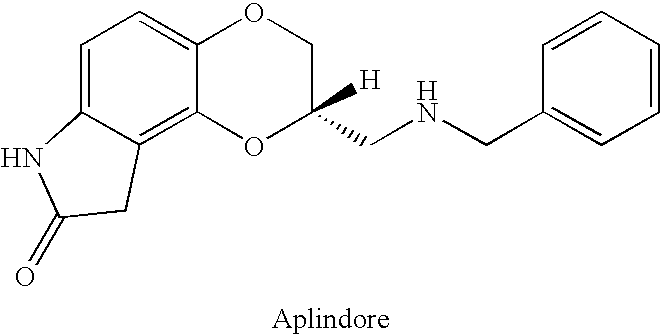
The present invention provides controlled release dosage formulations of compounds having the Formula: or pharmaceutically acceptable salts thereof, and in particular, aplindore. The dosage forms are useful, inter alia, for reducing side effects from administration of such compounds.
Owner:WYETH LLC
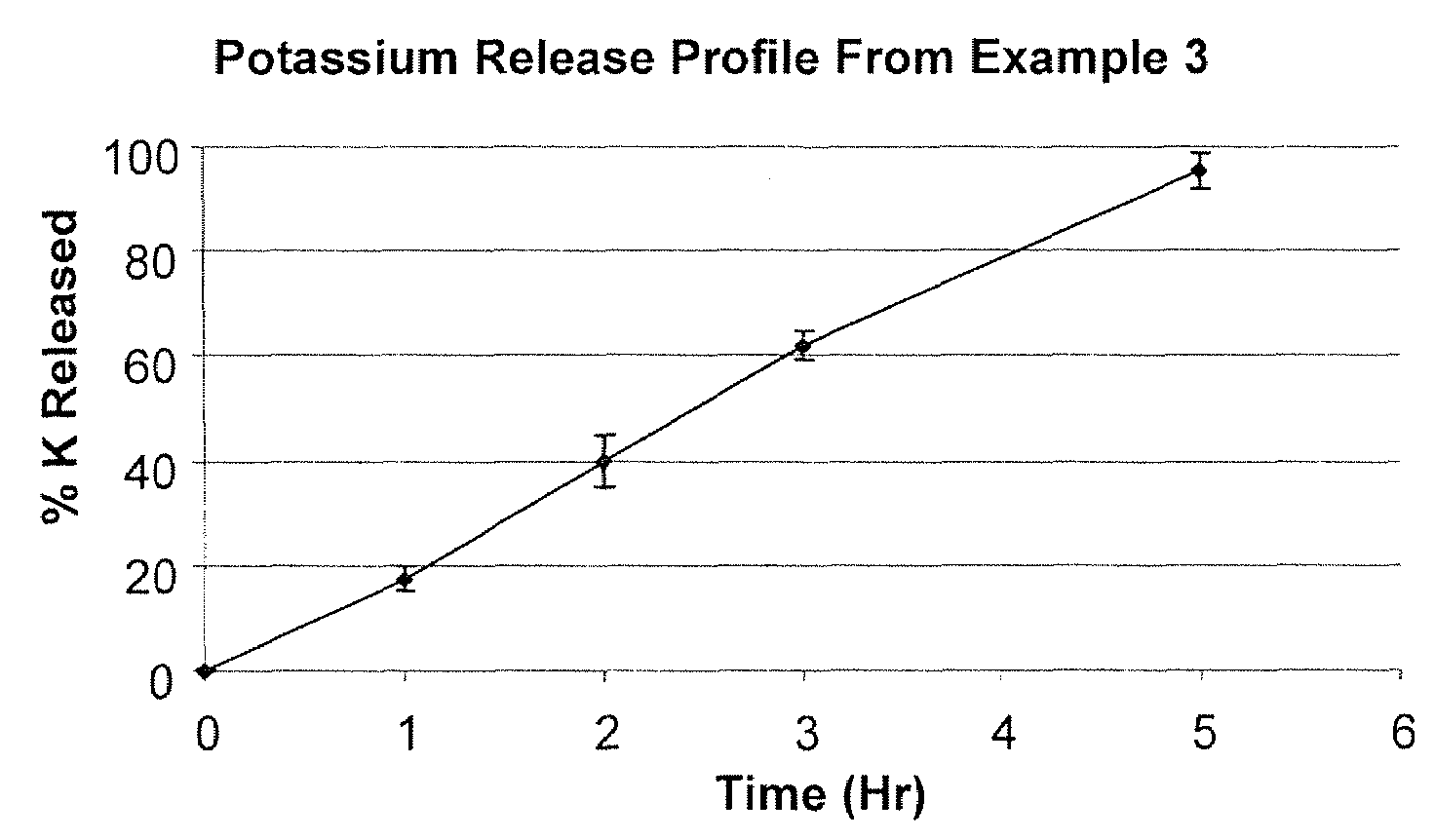
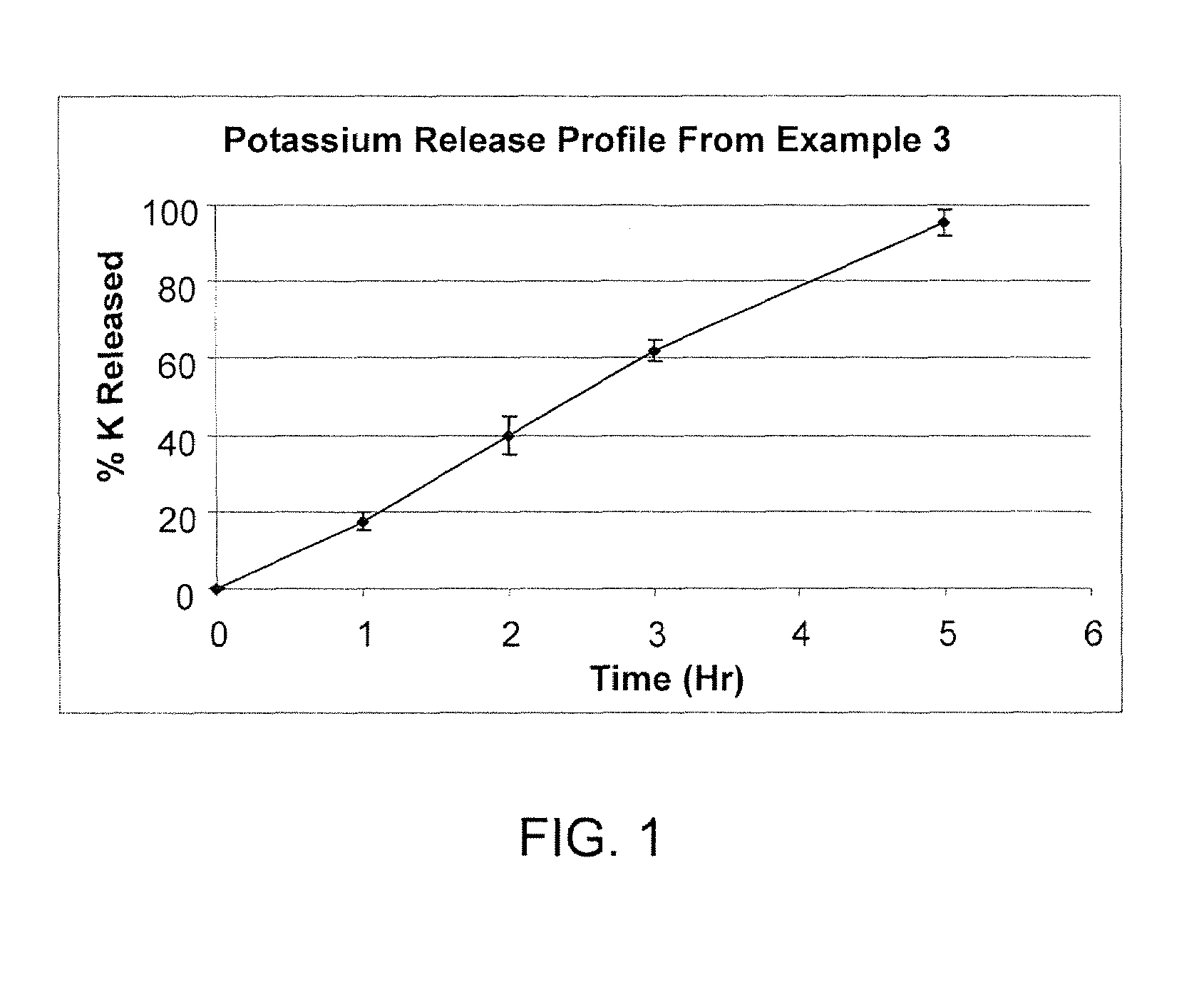
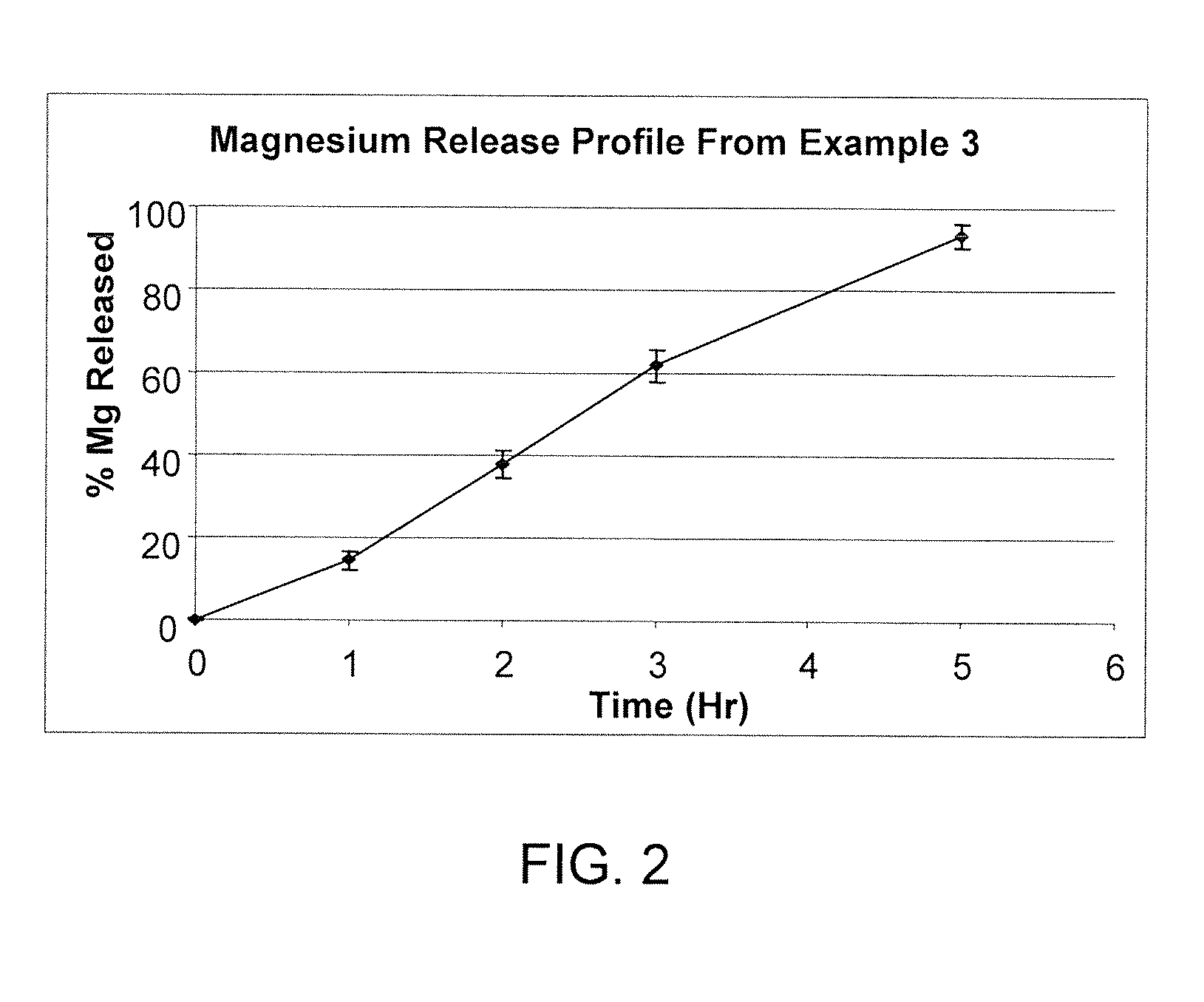
Short Term Slow Release Drug Delivery System
The present invention is directed to a novel short term slow release drug delivery system, preferably for solid oral dosage forms of water-soluble, alkaline salts of alkali metals and alkaline earth metals comprising polyvinylpyrrolidone and CPAA and preferably a wax component.
Owner:MISSION PHARMACAL CO INC
Precursor suspension of lyotropic liquid crystal and preparation method thereof
ActiveCN103040741AHigh viscosityHigh strengthSolution deliveryEmulsion deliveryOrganic solventUltimate tensile strength
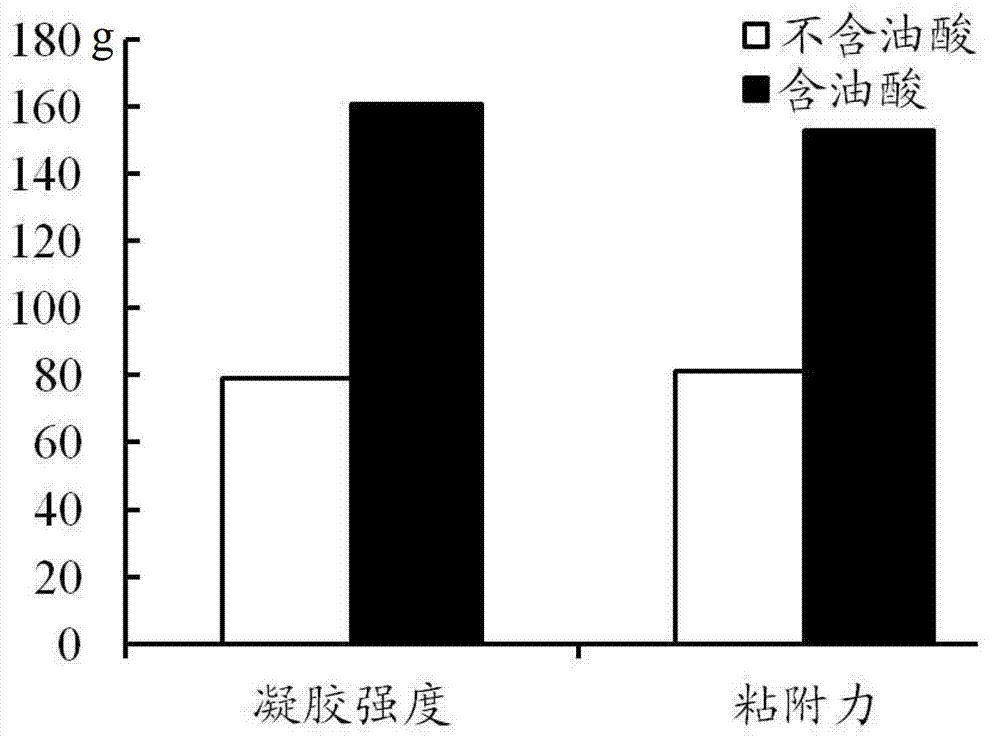
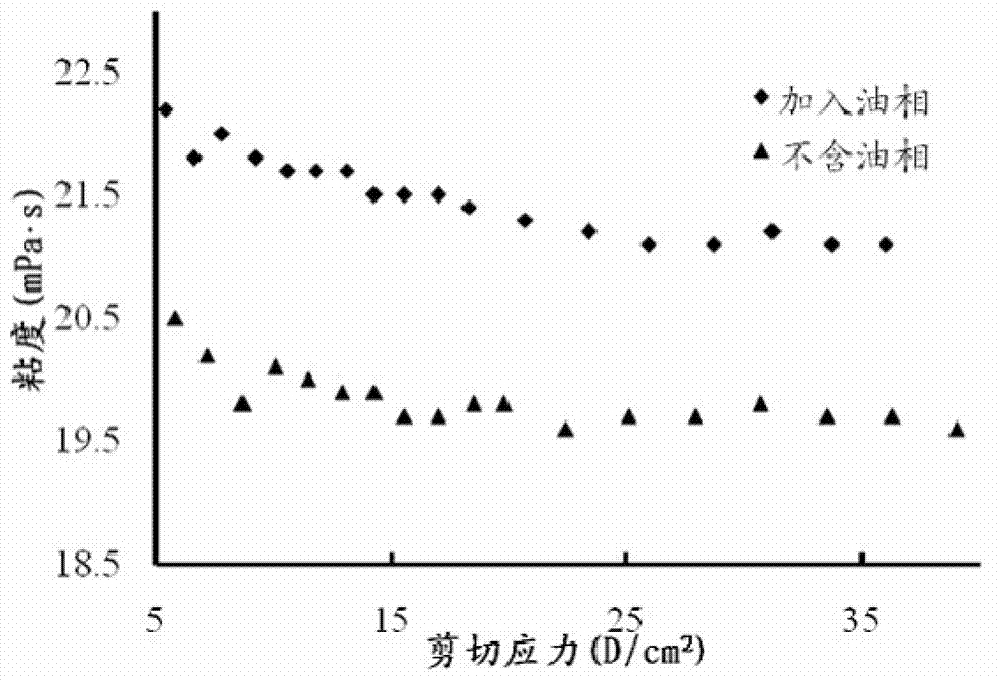
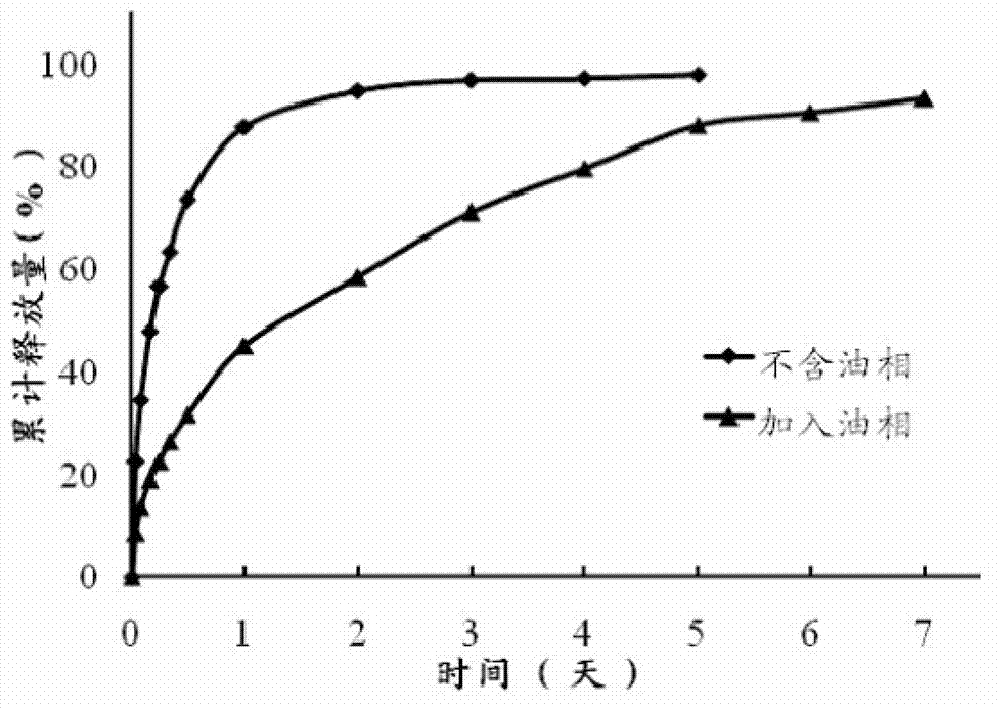
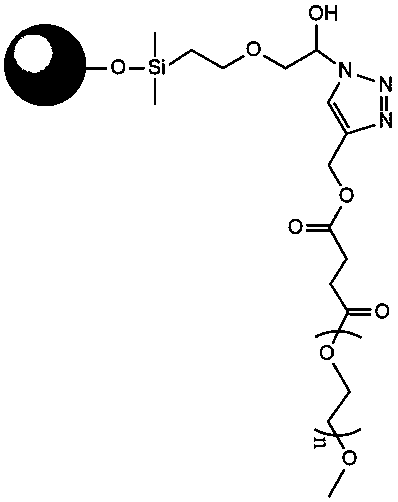
The invention discloses a precursor suspension of a lyotropic liquid crystal. The precursor suspension comprises lyotropic liquid crystal material, organic solvent, oil phase and a drug, wherein the weight percentage of the oil phase in the precursor suspension is 2-50 percent, the weight percentage of the drug in the precursor suspension is 1-30 percent, and the weight ratio of the lyotropic liquid crystal material and the organic solvent in the precursor suspension is 2-9:1. According to the invention, through the adding of the oil phase into the precursor suspension, the stability of the suspension is improved, the sedimentation rate is reduced, and the strength and the adhesive force of the gel formed are enhanced at the same time; the gel formed in the body is more liable to stay at a lesion location and less liable to be relocated and the shape is less liable to be damaged by the mechanical motion of the body, so that the drug therapy can be located effectively; and the preparation technology is simple and the precursor suspension of the lyotropic liquid crystal is a partial slow-release drug delivering system provided with a favorable perspective.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Long-acting sustained-release medicaments for treating and renovating bone disease and preparation thereof
InactiveCN101244275AExtended release timeImprove adhesionInorganic non-active ingredientsSkeletal disorderMicrosphereBiocompatibility Testing
The invention discloses a long acting slow-release drug carrier material for therapy and repair of bone disease and preparation method; wherein, the components of the drug carrier material are 4-8 weight account of strontium-doped calcium polyphosphate, 1-3 weight account of chitosan drug-loaded microspheres and 1-3 weight account of chitosan; the preparation method of the compound drug carrier material for therapy of osteomyelitis and other bone diseases is that (1) the chitosan-acetic acid solution is prepared, (2) the strontium-doped calcium polyphosphate powder is put into the chitosan-acetic acid solution and dispersed evenly, (3) the prepared chitosan drug-loaded microspheres is added, (4) the cross-linking agent is added for cross-linking, (5) freezing at temperature of 2-6 DEG C is processed for 12-48 hours, and (6) heating and drying are processed at temperature of 40-100 DEG C. The drug carrier material has the advantages of being applicable to therapy and repair of osteomyelitis and other bone diseases, releasing antibiotic for a long period, bone repair function, good biocompatibility and degradability, and ideal double function of therapy and repair of osteomyelitis and other bone diseases.
Owner:SICHUAN UNIV
The preparation method of the radiant crosslink porous reticular cells amnionic hydro gel dressing and its preparation method
InactiveCN1943796AOvercome the shortcomings of low mechanical strength and poor complianceConvenient for clinical operationAbsorbent padsBandagesFreeze-dryingPolyvinyl alcohol
The invention discloses a preparation method of an irradiative crosslink network cellular porous hydrogel dressing of amniotic membrane, the specific methods are: put the hydrogel solutions comprising of PVA, chitosan and acrylic acid-acrylamide copolymer on the porous stainless steel plate used to put the netty cellular amniotic membrane, add a small amount of agar, agitate uniformly, evacuate, and the hydrogel prototype was obtained; then press the hydrogel to film; then expose the membrane to 60Coy- ray irradiation in room temperature to crosslink, then dehydrate, freeze-dried, radiative sterilized, packed under aseptic conditions. The invention possesses good protection treatments, such as alleviate pain, resist to phlogosis and bacterium, and promote healing. It also has good hygroscopic properties, water retention, permeability and flexibility, further more we can have a selection of drugs according to sensitive bacteria of wound, pain, the need of arristing or activzte bleeding to and to help achieve sustained-release drug treatment purposes.
Owner:关志广
Extended release pharmaceutical dosage forms of carbidopa and levodopa and process of preparation thereof
The present invention relates to an extended-release pharmaceutical dosage form of carbidopa and levodopa comprising (i) an immediate-release unit of carbidopa and levodopa; (ii) an extended-release unit of carbidopa and levodopa; and (iii) an immediate or extended-release unit of a carboxylate salt. The present invention further provides a process of preparation thereof.
Owner:RANBAXY LAB LTD
Drug-loaded silica embolism microsphere and preparation method thereof
InactiveCN103751857AUniform and stable dispersionGood effectSurgeryPharmaceutical non-active ingredientsDiseaseHepatic tumor
Owner:TONGJI UNIV
Biosynthetic functional cellulose (BC) fibers as surgical sutures and reinforcement of implants and growing tissue
Embodiments of the invention are based on the fermentation of bacteria to produce nano-cellulose in oxygen permeable tubular bioreactors. The resulting hydrogel non-hollow fiber can be stretched and dewatered to form strong, stiff yet flexible fiber. The fiber can be dehydrated by freeze drying or solvent exchange to form macroporous material and then optionally soaked with a solution of growth factors, anti-inflammatory drugs, and / or anitibacterial agents to provide a slow release drug delivery device in fiber form. The surface of the fiber is composed of nano-structured cellulose which promotes cell migration, tissue integration, and the healing process. BC fibers are not degraded in the human body and thus are well suited as reinforcement of implants and growing tissue. Uses for the BC fibers include surgical sutures, and reinforcing and promoting regeneration of damaged tissue or implants.
Owner:BC GENESIS
Long acting slow releasing drug addiction eliminating prepn and its prepn process and use
ActiveCN1973840ASolve the sudden release problemRelease stabilityOrganic active ingredientsNervous disorderMicrosphereSolvent
The present invention provides the preparation process and usage of slow released naltrexone (NTX) preparation, which is used in the rehabilitation after eliminating opium addiction. The NTX preparation includes NTX as the opium receptor antagonist and matrix of biodegradable polymer material polylactic acid. The preparation process includes emulsification and solvent volatilization to prepare microsphere, pressing into tablet, coating with polylactic acid and other steps. The NTX preparation has great medicine carrying amount and high encapsulating rate, and may reach blood medicine concentration for over 360 days. When it is used, the NTX preparation is injected with special injector into subcutaneous fat for NTX to release slowly and persistently to maintain the effective blood medicine concentration.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Biodegradable cardia support
InactiveCN102973340AAvoid the hassle of taking outPrevent proliferationStentsSurgeryAntiinflammatory drugDrug release
The invention provides a biodegradable cardia support. The biodegradable cardia support comprises a biodegradable support framework which is formed by weave of biodegradable high polymer material filaments, and a layer of controlled-release drug layer is coated on the surface of the biodegradable support framework. The support framework and drug carriers are made of high polymer biodegradable materials and can fully and automatically degradate after time window treatment is finished, and therefore the trouble of taking out the support is avoided. In addition, the controlled-release drug layer can release anti-inflammatory drugs stably through drug controlled-release technology, and therefore fibroblast hyperplasia caused by esophagus repair reaction can be effectively restrained, subsequent scar tissue hyperplasia and thickening can be reduced, and tissue hyperplasia on the surface of the support after the support is implanted is restrained. Thus, the time window of support implantation treatment can be prolonged, supporting force of the support on tube walls can be increased, and long-dated reappearance can be reduced.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL +1
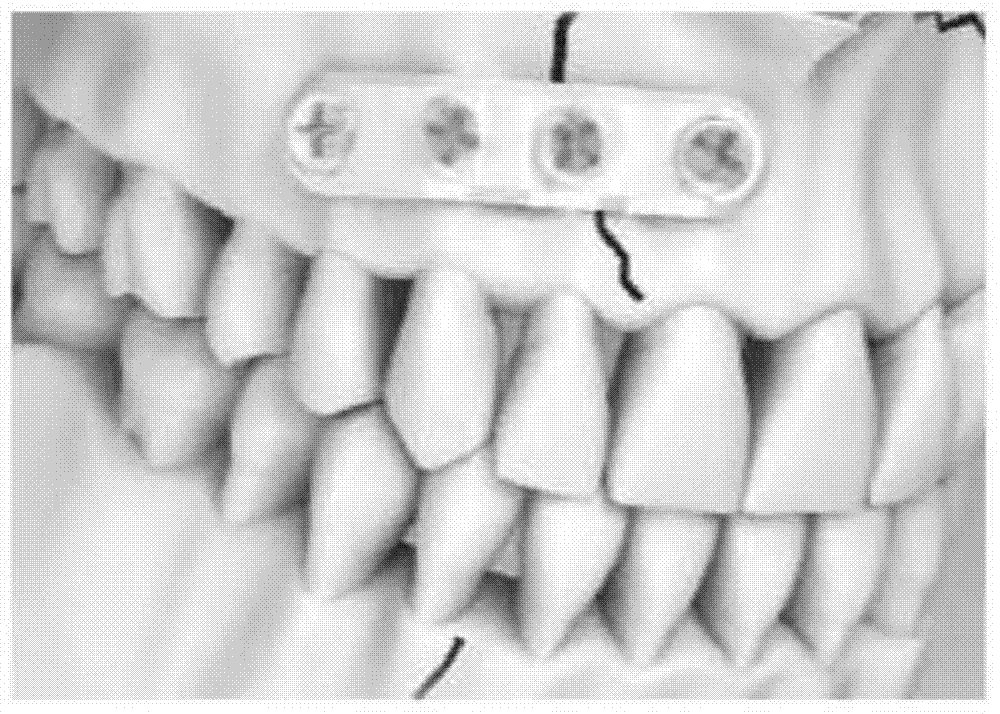
Intraosseous fixation implant and preparation method thereof
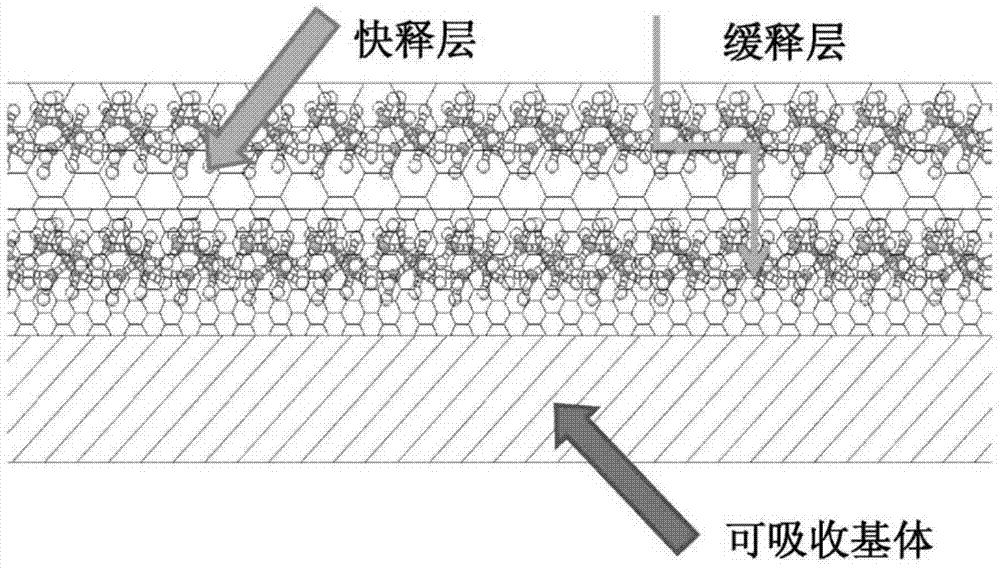
InactiveCN104740692ALong-term sustained-release drug effectQuick releaseSurgeryCoatingsDrug coatingBiodegradable polymer
An intraosseous fixation implant and a preparation method therefor. In the intraosseous fixation implant, a biodegradable polymer material is used as a substrate, a drug coating is loaded on the surface of the substrate, the drug coating has a double-layer structure, the first layer close to the substrate is a slow-release drug layer, and the second layer on the outer side of the first layer is a quick-release hydrophilic layer. The intraosseous fixation implant has triple functions of being antibacterial, absorbable and fixable.
Owner:SHANGHAI MICROPORT ORTHOPEDICS +1
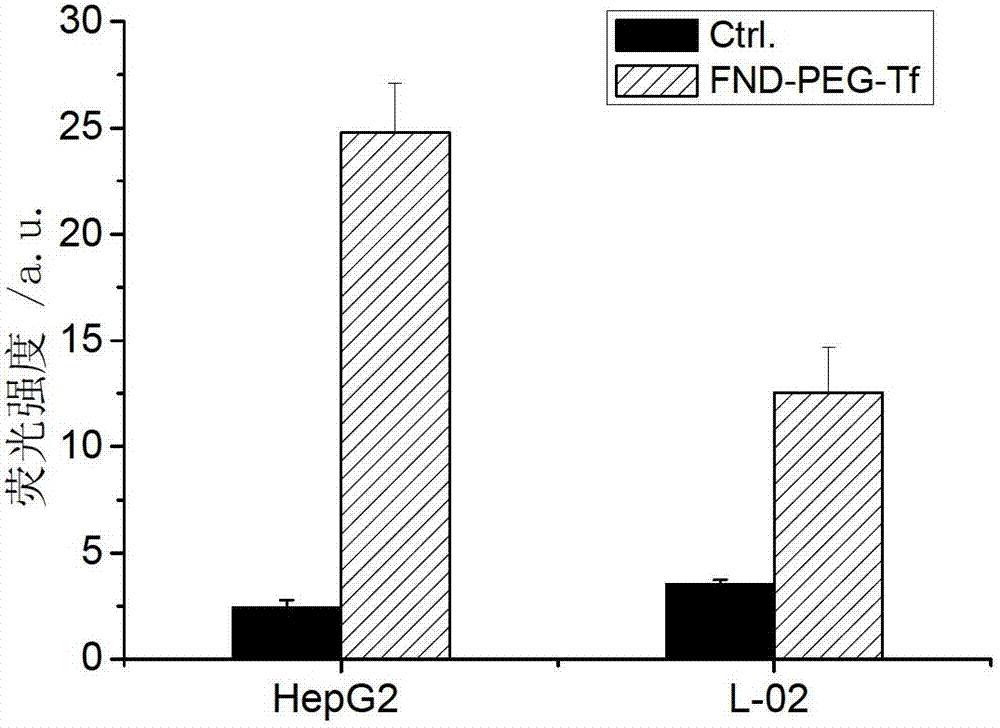
Targeting nano diamond carrier and targeted drug, and preparation method and application thereof
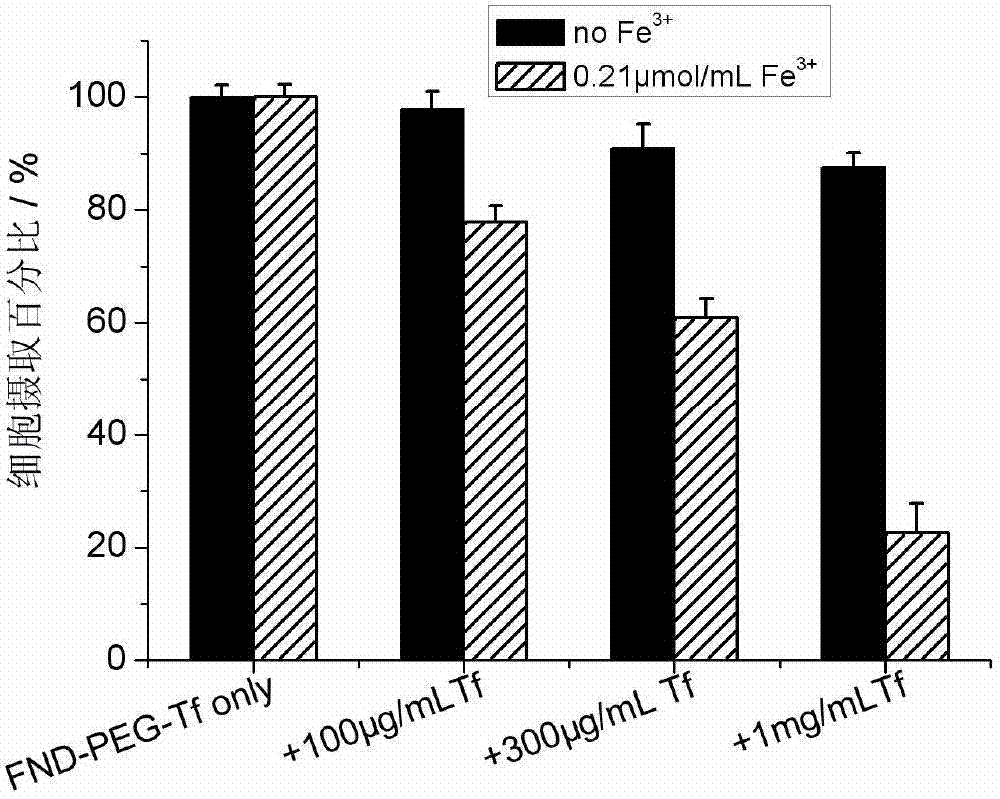
InactiveCN103203024AGood biocompatibilityNo toxicityOrganic active ingredientsAntiinfectivesSide effectCancer cell
The invention provides a targeting nano diamond carrier ND-PEG-Tf and a preparation method, and a targeted nano-drug ND-PEG-Tf-DOX prepared by loading chemotherapeutic adriamycin through physical absorption. The carrier interacts with a human liver cancer cell (HepG2) and a normal liver cell (L-02) and free transferrin is taken as a competitor, and the result indicates that the ND-PEG-Tf carrier is mediated by a transferrin receptor into the cells; MTT testing of interaction of ND-PEG-Tf-DOX with the two cells indicates that the nano-drug has targeted anti-tumor effect on cancer cells; and compared with the cell damage force of independent adriamycin, ND-PEG-Tf-DOX has the characteristics of a slow-release drug. Therefore, ND-PEG-Tf-DOX is capable of either reducing toxic and side effects of chemotherapeutics on normal cells or improving the damage force of the chemotherapeutics on tumor cells. In addition, the targeting nano diamond carrier can be applied to preparing targeted anti-tumor drugs.
Owner:SHANXI UNIV
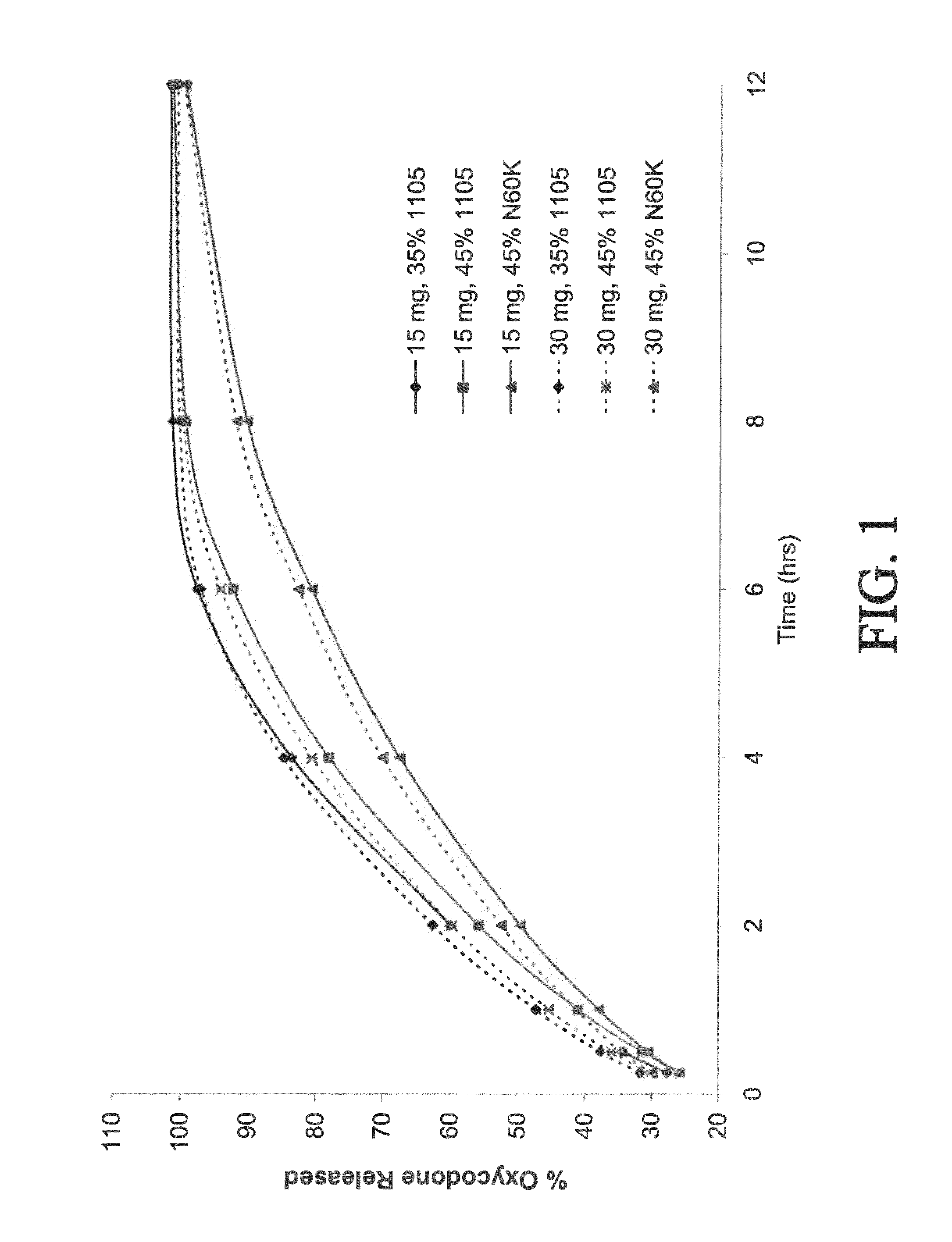
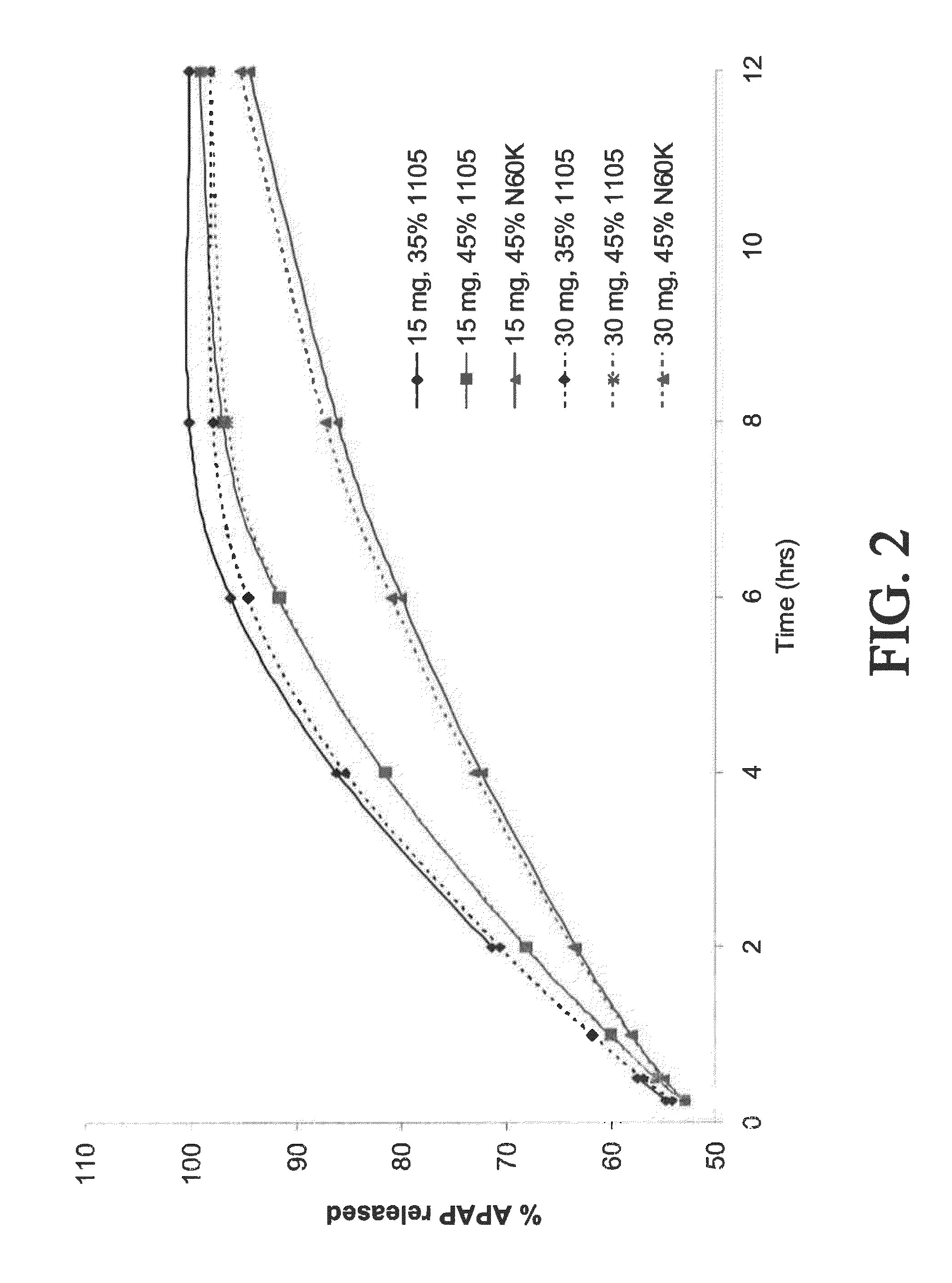
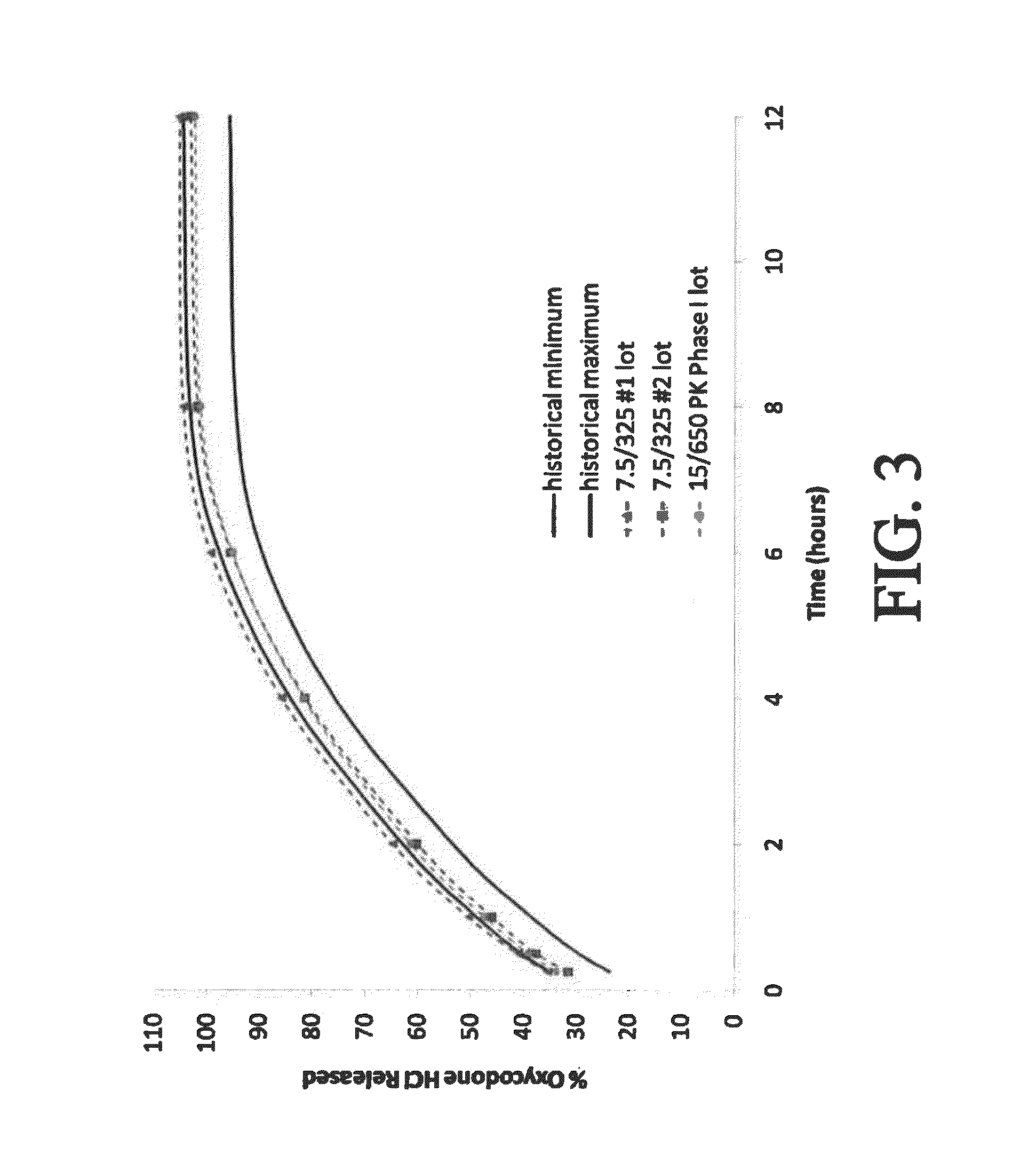
Gastric retentive extended release pharmaceutical compositions
The present disclosure provides extended release pharmaceutical compositions comprising an opioid and an additional active pharmaceutical ingredient, wherein the composition exhibits gastric retentive properties which are achieved by a combination of a physical property of the composition and release of the opioid, wherein upon administration to a subject, the composition has at least one pharmacokinetic parameter that differs by less than about 30% when the subject is in a fasted state as compared to a fed state.
Owner:MALLINCKRODT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com