Intraosseous fixation implant and preparation method thereof
A technology of implants and drugs, applied in the field of medical devices, can solve problems such as poor blood supply, difficult to sterilize drug concentration, etc., and achieve the effect of rapid release in the early stage, long-term sustained release drug effect, and elimination of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
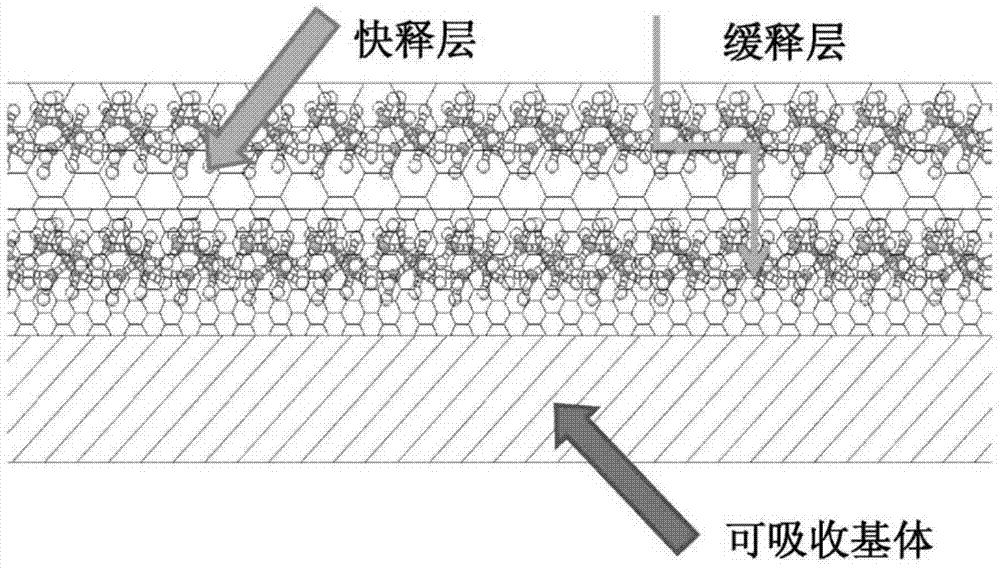
[0035] Absorbable rods are prepared by compression molding and then machined into bone screws. Prepare a mixed solution of polylactic-co-glycolic acid (PLGA) and vancomycin (the ratio of PLGA to vancomycin is 10:1), put it into the spray paint tank of the spray testing machine and spray it on the bone plate prepared in the previous process Above, the atomization pressure is 11psi, the spraying time is 40s, the interval time is 5min, and the number of spraying is 12 times. The second layer (surface layer) is to prepare a quick-release hydrophilic layer. The carrier uses hyaluronic acid, equipped with a hyaluronic acid aqueous solution with a concentration of 0.1%, and the mass ratio of drug to hyaluronic acid is 1:10. The time is 30s, the interval time is 4min, and the number of spraying is 10 times. After spraying, dry in a vacuum oven until the sample has a constant weight.
Embodiment 2
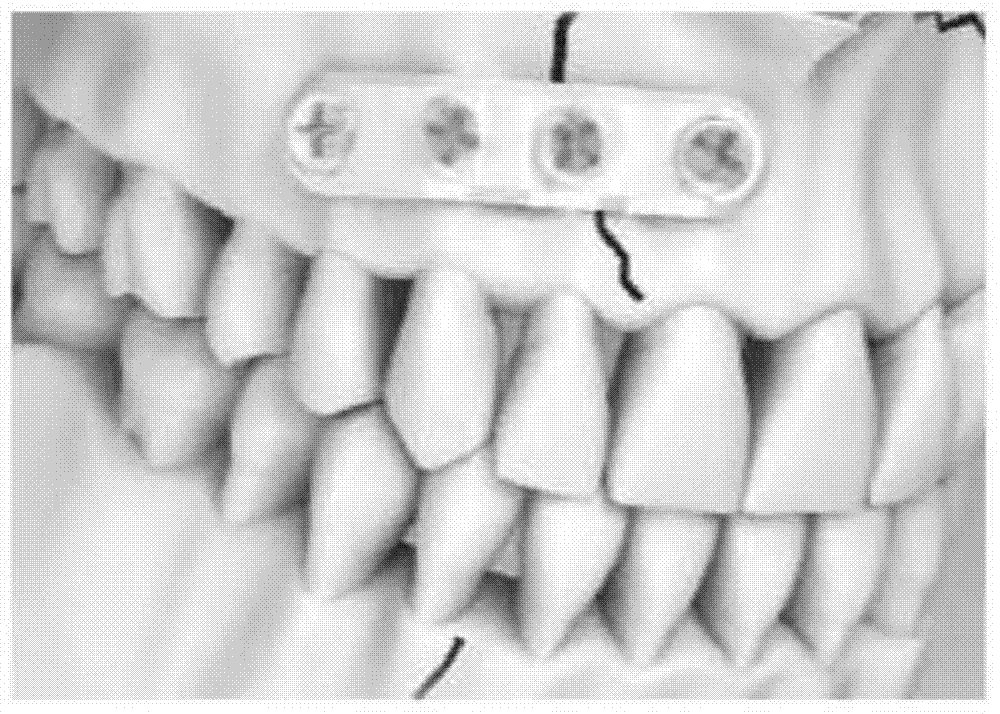
[0037] Absorbable plates are prepared by compression molding and then machined into small four-hole plates (e.g. figure 2 shown). Prepare a mixed solution of polylactic acid and gentamicin (the ratio of polylactic acid and gentamicin is 2:1), put it into the spray paint tank of the spray testing machine and spray it on the bone plate prepared in the previous process, the atomization pressure 10psi, spraying time 20s, interval time 3min, spraying times 10 times. The second layer (surface layer) is to prepare a quick-release hydrophilic layer. The carrier uses hyaluronic acid, equipped with a hyaluronic acid aqueous solution with a concentration of 0.1%, and the mass ratio of drug to hyaluronic acid is 1:10. The time is 30s, the interval time is 4min, and the number of spraying is 15 times. After spraying, dry in a vacuum oven for 3 days until the sample has a constant weight.
Embodiment 3
[0039]Prepare absorbable screws by injection molding technology, then prepare a mixed solution of polylactic acid and tobramycin (the ratio of polylactic acid and tobramycin is 3:1), put it into the spray paint tank of the spray testing machine and spray it to the previous process for preparation On a good bone nail, the atomization pressure is 12psi, the spraying time is 20s, the interval time is 5min, and the number of spraying times is 15 times. The second layer (surface layer) is to prepare a quick-release hydrophilic layer. The carrier is gelatin, equipped with a gelatin aqueous solution with a concentration of 0.15%, and the mass ratio of drug to gelatin is 1:10. The coating rate is 20 mm / min, the number of dip coatings is 3 times, and the interval time is 30 minutes. After the dip coating is completed, it is dried in a vacuum oven for 3 days until the sample has a constant weight.
[0040] Effect example
[0041] The absorbable bone plate of the present invention has a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com