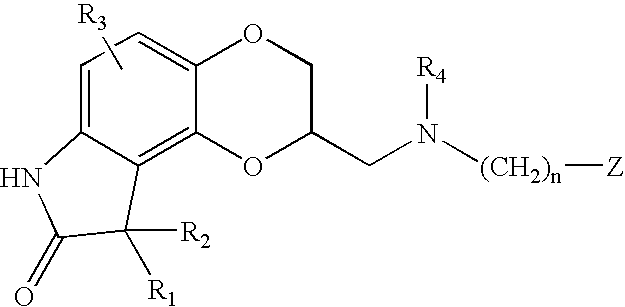
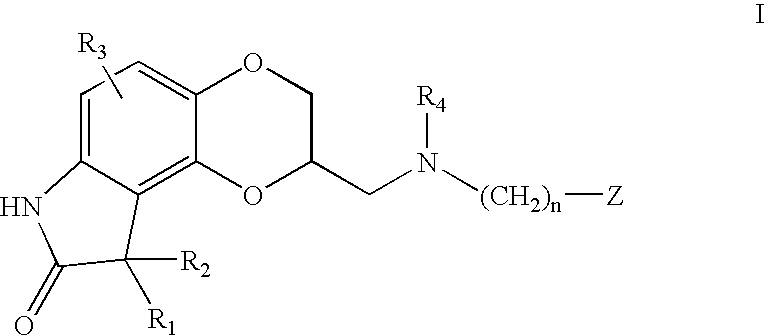
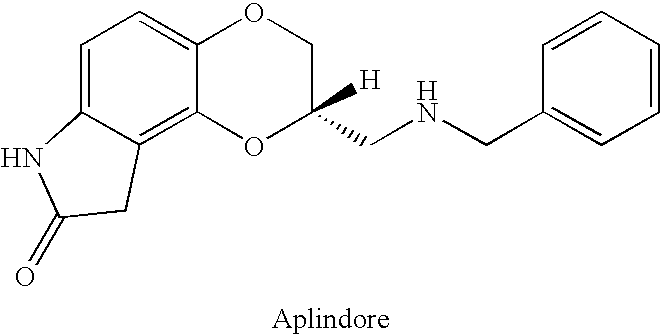
Sustained release pharmaceutical compositions
a technology of pharmaceutical compositions and suspensions, which is applied in the direction of drug compositions, heterocyclic compound active ingredients, biocide, etc., can solve the problems of high initial drug concentration and failure to maintain, and achieve high viscosity, low viscosity, and high viscosity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sustained Release Aplindore Fumarate Pellets Prepared Using Extrusion / Spheronization and SR Coating
[0107] Batches were made by blending drug with Microcrystalline Cellulose (Avicel PH102) and granulating with water to form a wet mass. The wet mass was extruded through a 1.0 mm screen and spheronized on a small Caleva system. The formulations and the dissolution data in 0.1 N HCl for the uncoated pellets are presented in Table 1. The Avicel matrix does not retard the release of aplindore and essentially all of the drug is released from the uncoated pellets in 30 minutes.
TABLE 1Formulation and Dissolution of Uncoated PelletsComposition12Aplindore (% listed as free5.0%10.0%base)Avicel PH 10295.0%90.0%Dissolution (Hrs)% Dissolved% Dissolved0.1 N HCl0.2576.9185.900.591.1290.30194.7991.07294.1390.86
[0108] The spheres prepared by extrusion / spheronization were coated with Eudragit RS / RL and Surelease to control the release of aplindore. The formulation and the dissolution profiles are sh...
example 2
Sustained Release Aplindore Fumarate Pellets Prepared By Sustained Release Coating of Layered Sugar Spheres
[0109] Aplindore fumarate sustained-release pellets were also prepared by layering the active drug on sugar spheres. The formulation and the dissolution data are given in Table 3. Composition 5 was tested for in vivo release in monkeys. The data is included in Table 4.
TABLE 3Formulation and dissolution of sustained release coated aplindorelayered sugar spheres.Composition5% W / WSpheroids coreSugar spheres 25 / 30 mesh100Drug coating (5% as free base)aplindore fumarate44.4HPMC 6 cps55.6Controlled release CoatingEudragit RS10045.5Triethylcitrate9.1Talc45.5Dissolution Time(Hr)% Dissolved 0.1 N HCl 0.250.9 0.53.77 113.71 231.1pH 6.8 phosphate 18.15 221.27 438.37 863.891279.02
[0110]
TABLE 4Summary Aplindore Bioavailability Parameters in MonkeysAUC% RelativeCom-(ng * hr / CmaxTmaxBio-positionDescriptionmL)(ng / mL)(hr)availability6Instant Release1290166.82.5100capsule3Surelease coated369....
example 3
Sustained Release Tablets Prepared Using Co-Compression Method
[0111] The use of a co-compressed tablet was investigated for the purpose of having a zero-order or nearly zero-order release profile. Different release profiles could be obtained by varying the amounts of drug and polymer (HPMC) in the core tablet and in the outer compressed coat, as well as the total tablet weight. All tablets were made individually on the Carver press using standard round tooling. The formulations and the dissolution data are given in Table 5. In vivo absorption data in Monkeys for Composition 8 are included in Table 4.
TABLE 5Formulations for Aplindore Fumarate Co-CompressedTablets (Tablet-in-Tablet):8INGREDIENTS:InnerOuterAplindore (as base)5.0005.000Lactose, Spray dried25.80068.250Avicel, PH 10125.80068.250Methocel K4M Prem CR36.00048.000Methocel K100 Prem LV7.00059.500Mg-stearate0.4001.000Total100.000250.000Dissolution (Hrs)% DissolvedpH 6.8 phosphate buffer 0.255.50 0.508.47 112.98 112.98 219.70...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com