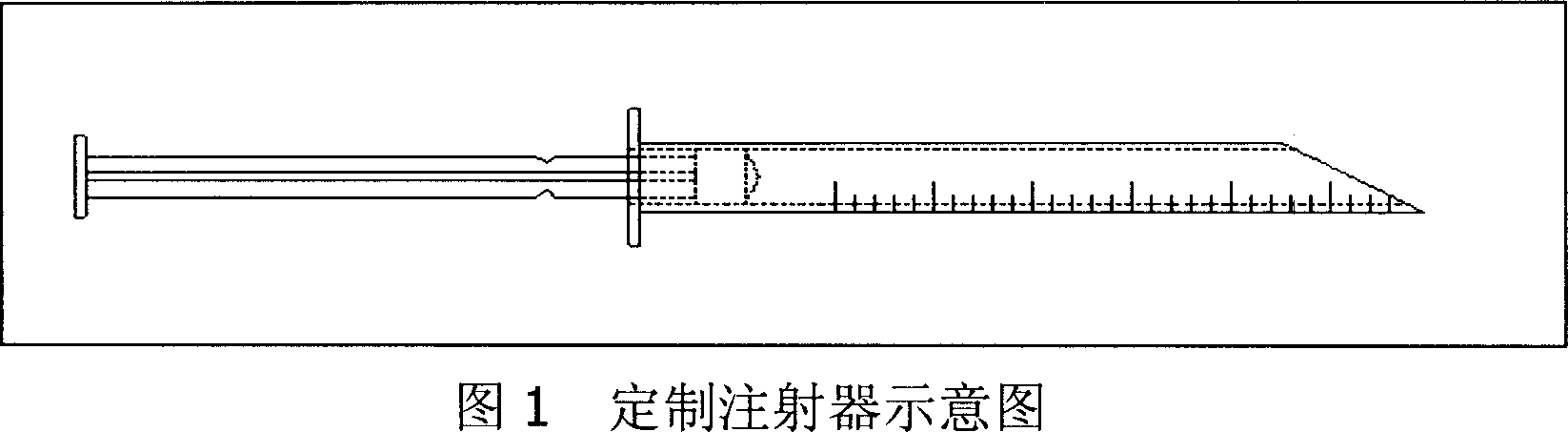
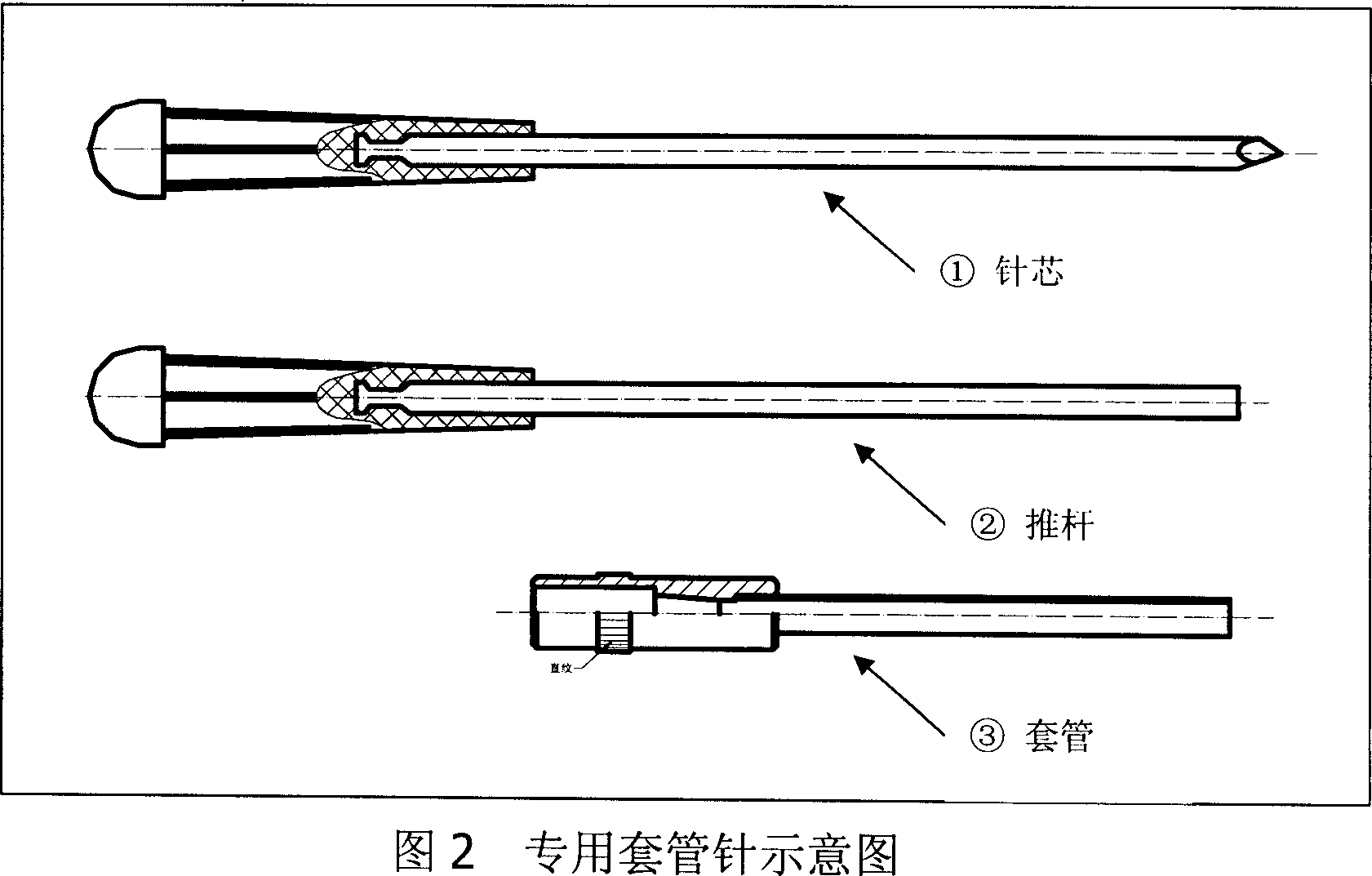
Long acting slow releasing drug addiction eliminating prepn and its prepn process and use
A technology of sustained-release preparations and sustained-release agents, applied in the field of long-acting naltrexone sustained-release agents, to achieve the effect of sudden release of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
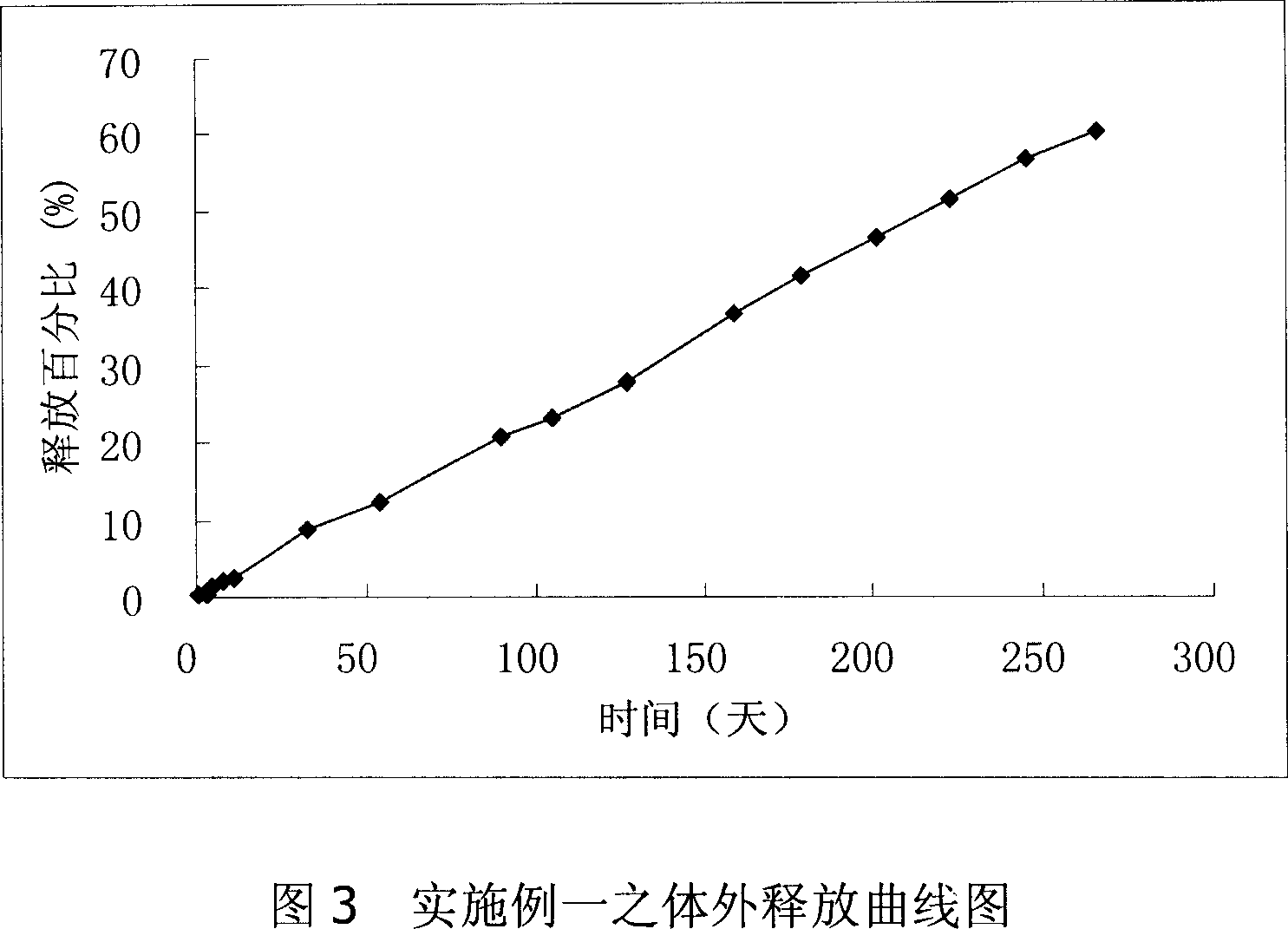
[0024] The degradable polymer material polylactic acid (L-PLA) in naltrexone sustained-release agent has a viscosity coefficient of 2.8dl / g (chloroform, measured at 30°C) and an average molecular weight of about 200,000 daltons. Dissolve polylactic acid (0.6g) and naltrexone (0.5g) in dichloromethane (5mL) to form an oil phase emulsion. Using emulsion emulsification and volatilization method, the emulsion is slowly added to 500mL 2.5% PVA solution. Continue mechanical stirring at room temperature until the dichloromethane is completely volatilized, and the stirring speed is 2500 rpm. The microspheres were collected by filtration, washed, and freeze-dried. The collected microspheres are 50-100 microns in size. Use appropriate pressure to compress the microspheres into tablets. The tablet diameter is 3.6mm and the hardness test size is 6kg. The second coating is performed, and the thickness of the coating is 0.1 ~0.3mm. Using the in vitro release test method, continuous measurement ...
Embodiment 2
[0026] The degradable polymer material polylactic acid (L-PLA) in the naltrexone sustained-release agent has a viscosity coefficient of 2.85dl / g (chloroform, 30°C) and an average molecular weight of about 210,000 Daltons. Dissolve polylactic acid (0.5g) and naltrexone (0.6g) in dichloromethane (5mL) to form an oil phase emulsion. Using emulsion emulsification and volatilization method, the emulsion is slowly added to 500mL 1.5% PVA solution , Continue mechanical stirring at room temperature until the dichloromethane is completely volatilized, and the stirring speed is 2000 rpm. The microspheres were collected by filtration, washed, and freeze-dried. The collected microspheres are 80-120 microns in size. Use appropriate pressure to compress the microspheres into tablets. The tablet diameter is 3.6mm and the hardness test size is 6kg. The second coating is performed, and the thickness of the coating is 0.1 ~0.2mm. Using the in vitro release test method, continuous measurement for 22...
Embodiment 3
[0028] The degradable polymer material polylactic acid (L-PLA) in the naltrexone sustained-release agent has a viscosity coefficient of 3.0dl / g (chloroform, measured at 25°C) and an average molecular weight of about 220,000 Daltons. Dissolve polylactic acid (0.5g) and naltrexone (0.5g) in dichloromethane (5mL) to form an oil phase emulsion. Using emulsion emulsification and volatilization method, the emulsion is slowly added to 500mL 1.5% PVA solution. Continue mechanical stirring at room temperature until the dichloromethane is completely volatilized, and the stirring speed is 2000 rpm. The microspheres were collected by filtration, washed, and freeze-dried. The collected microspheres are 80-150 microns in size and compressed into tablets with appropriate pressure. The tablet diameter is 5.5mm and the hardness test size is 8kg. The coating is processed once, and the thickness of the coating is 0.1-0.3mm. between. Using the in vitro release test method, continuous measurement for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com