Biological degradable polymer-solid liposome sustained-release storing system and preparation method thereof
A technology for degrading polymers and solid lipids, used in pharmaceutical formulations, drug delivery, peptide/protein components, etc., can solve problems such as being difficult to adapt to industrialized large-scale production, prone to inflammation and infection, and toxic organic solvent residues. The effect of sustained and smooth release, improved stability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Dissolve 10% (w / v) PLGA (molecular weight 10,000, monomer ratio 70:30) and 1% (w / v) risperidone in N-methylpyrrolidone, and then add 1% (w / v) / v) Solid lipid, shaken to form matrix.
Embodiment 2-11
[0036] Repeat the steps of Example 1 with different types of polymers, solid lipids, and organic solvents, as shown in the following table:
[0037] Table 1: Description of reservoir morphology composed of different types of polymers, solid lipids, and organic solvents.
[0038] implement
[0039] 70∶30)
Embodiment 12
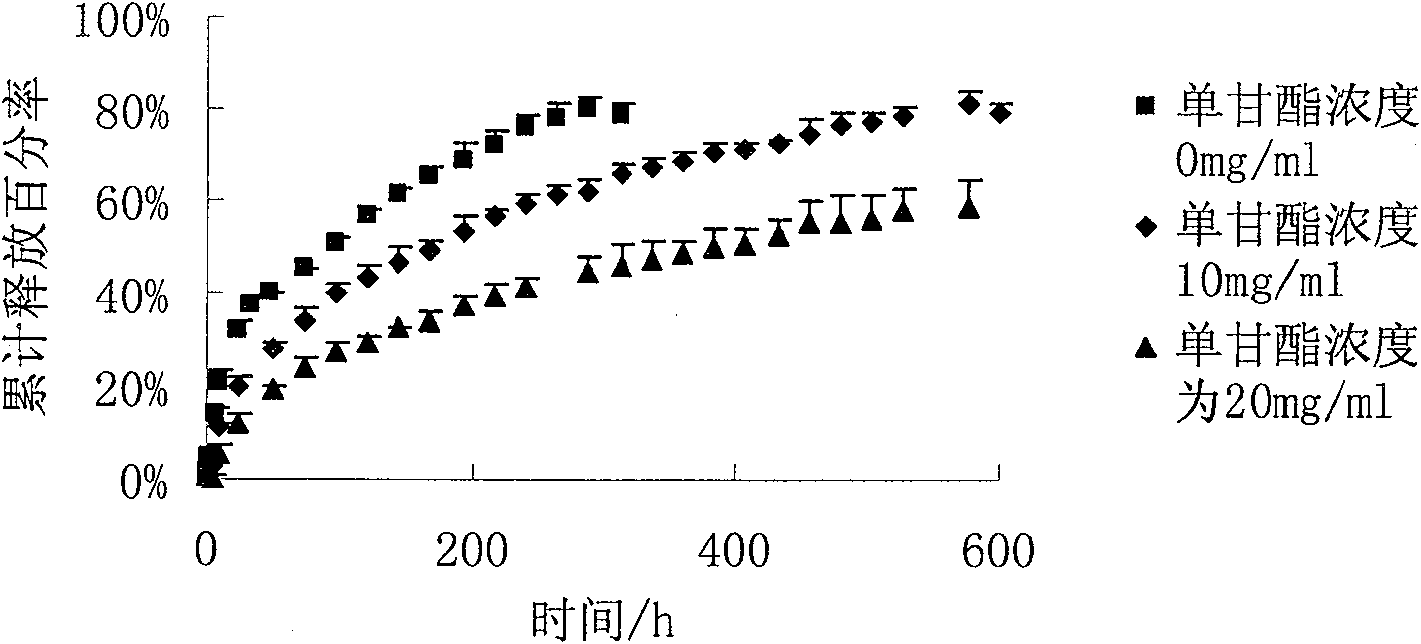
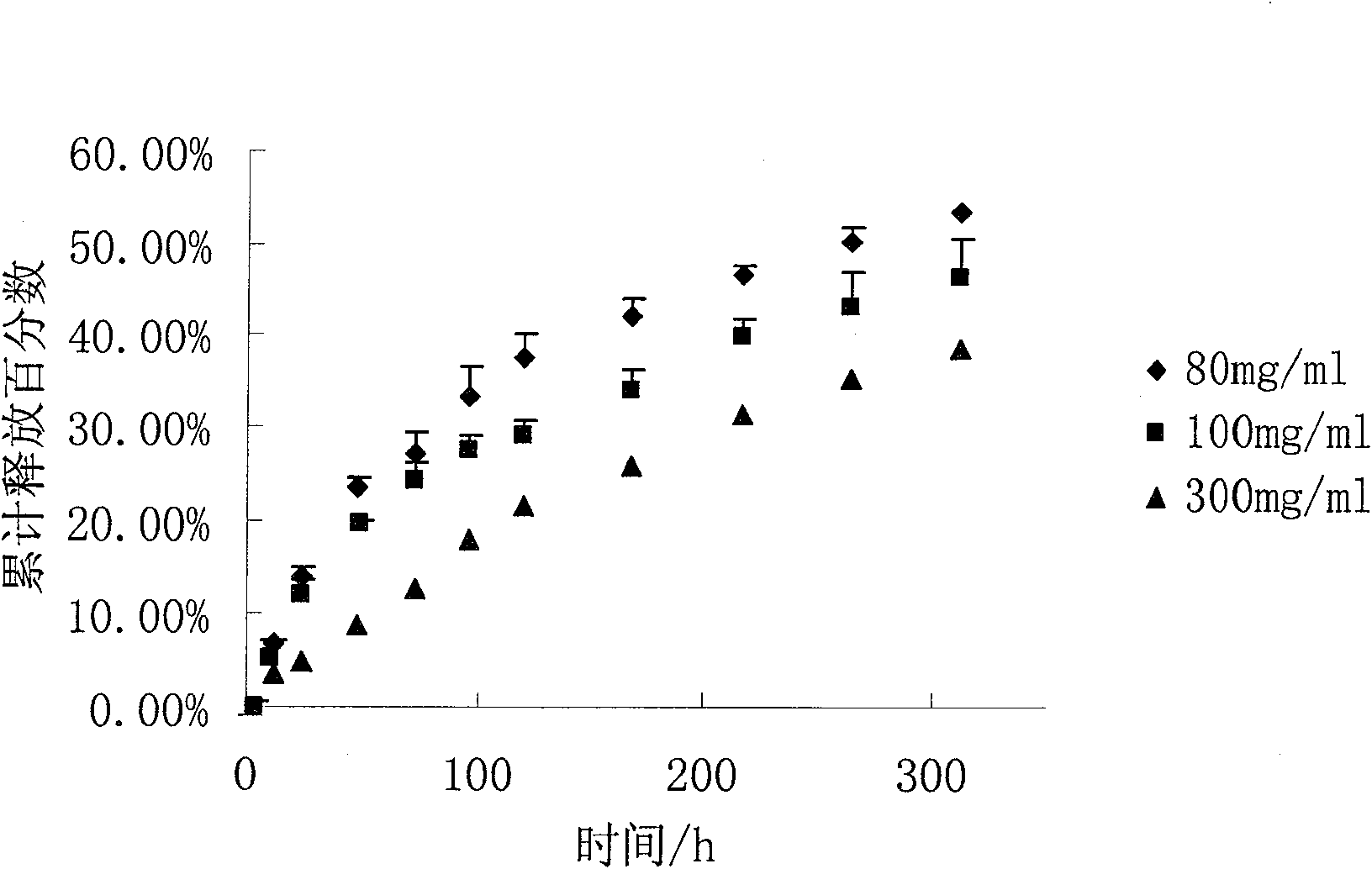
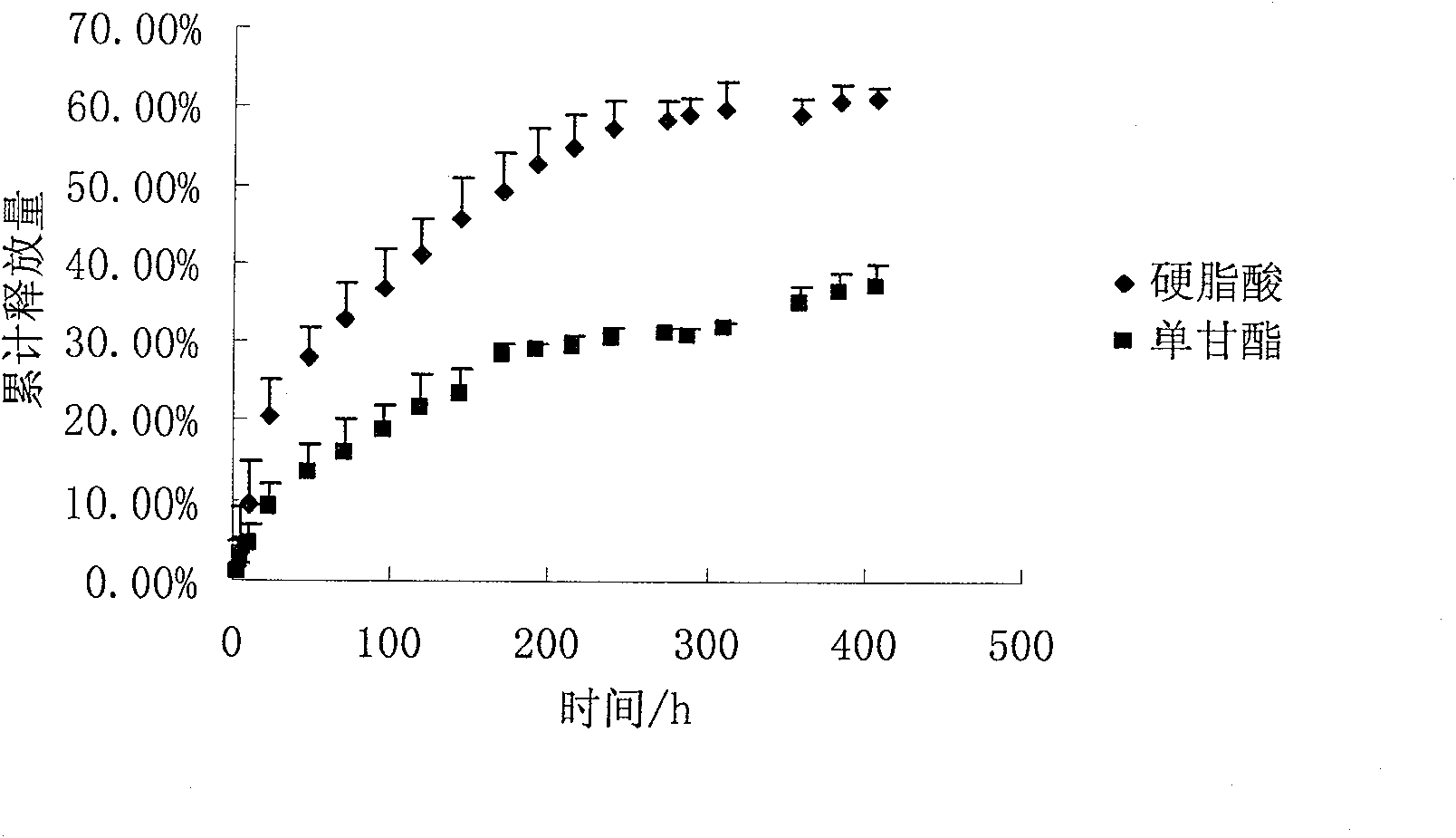
[0041] Get 100ml benzyl alcohol (BA) and 900ml benzyl benzoate (BB) and mix uniformly, accurately weigh 10g risperidone and 10g glyceryl monostearate and dissolve in the mixed solvent of benzyl alcohol and benzyl benzoate, and then Take 100 g of PLGA (70:30 molecular weight: 5w) and add it to the above mixed solution, shake at 37° C. until all dissolved to form a transparent and uniform solution.
[0042] In vitro release test conditions: take 1ml of the above homogeneous solution, transfer it into a dialysis bag, shake in 250ml of normal saline, and shake at 37°C for in vitro release test.
[0043] Results: The drug can be continuously and steadily released for 25 days, and the cumulative release amount is greater than 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com