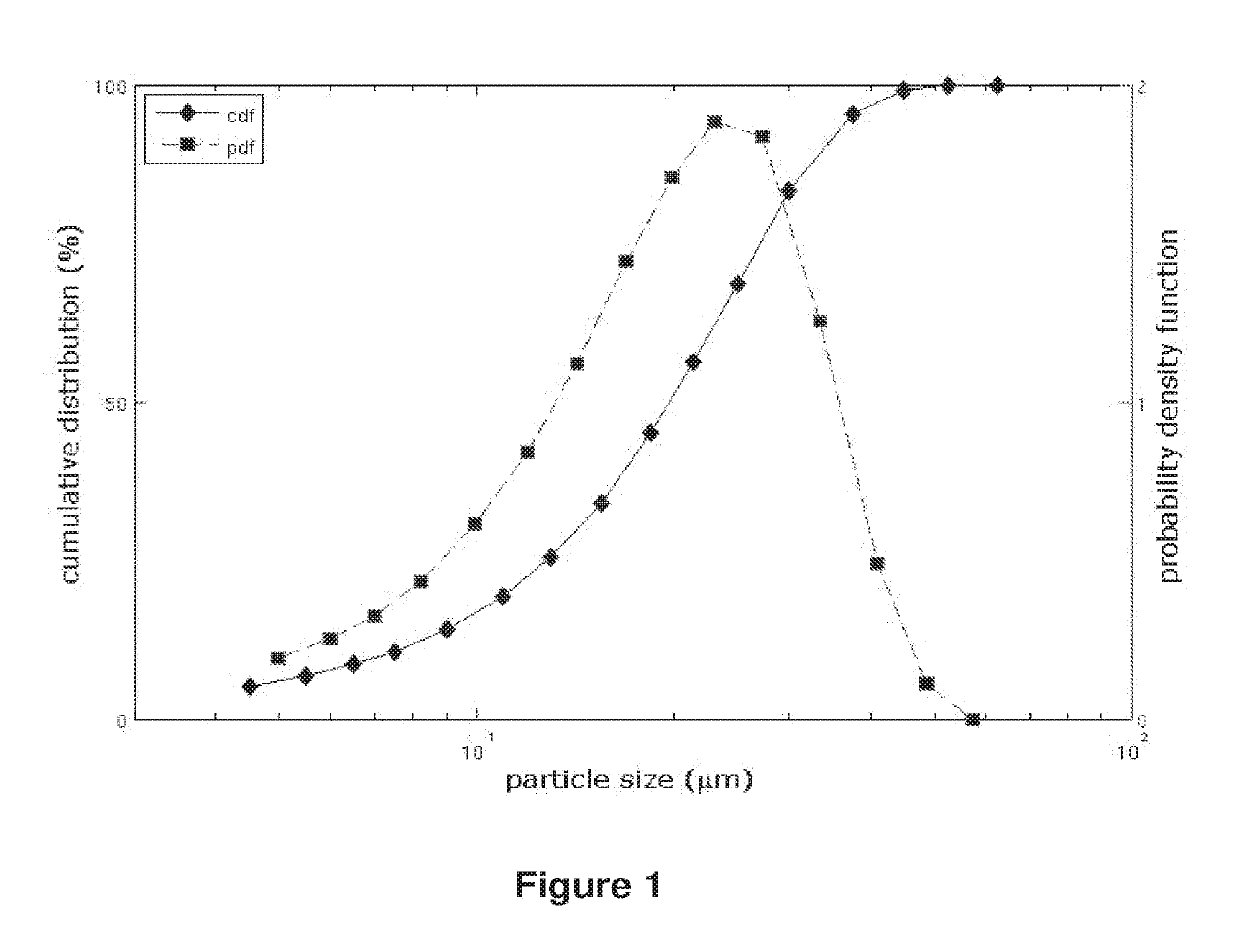
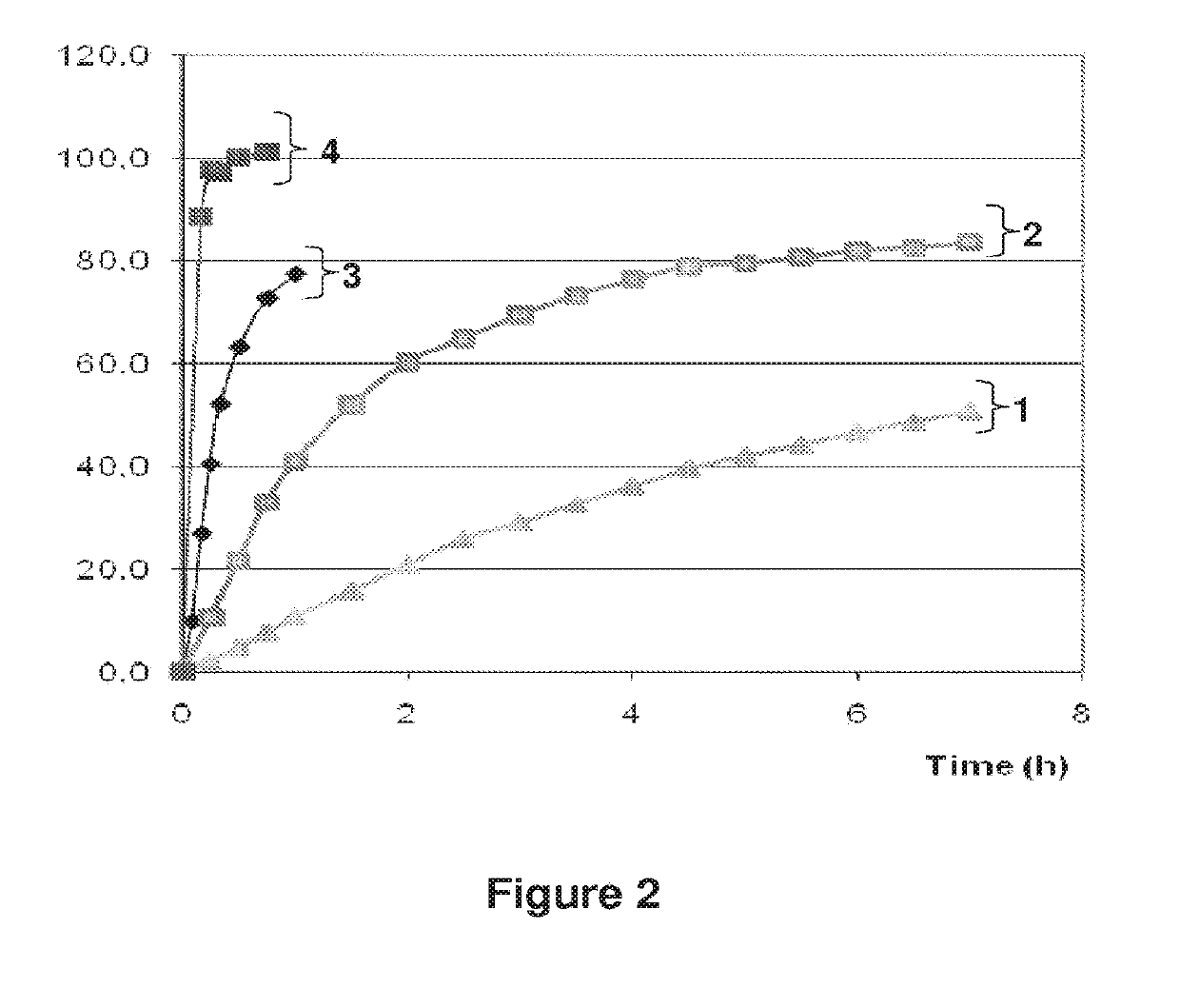
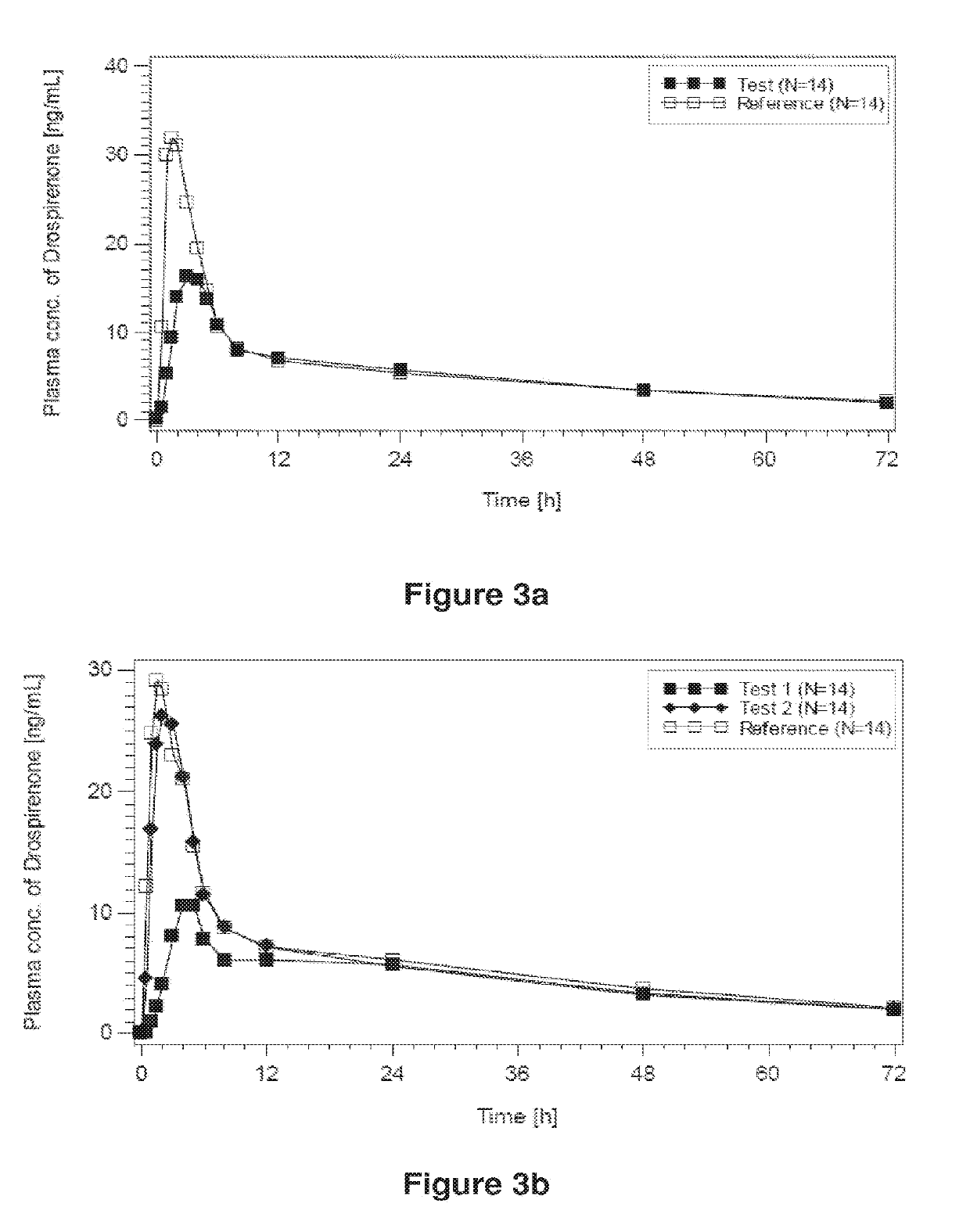
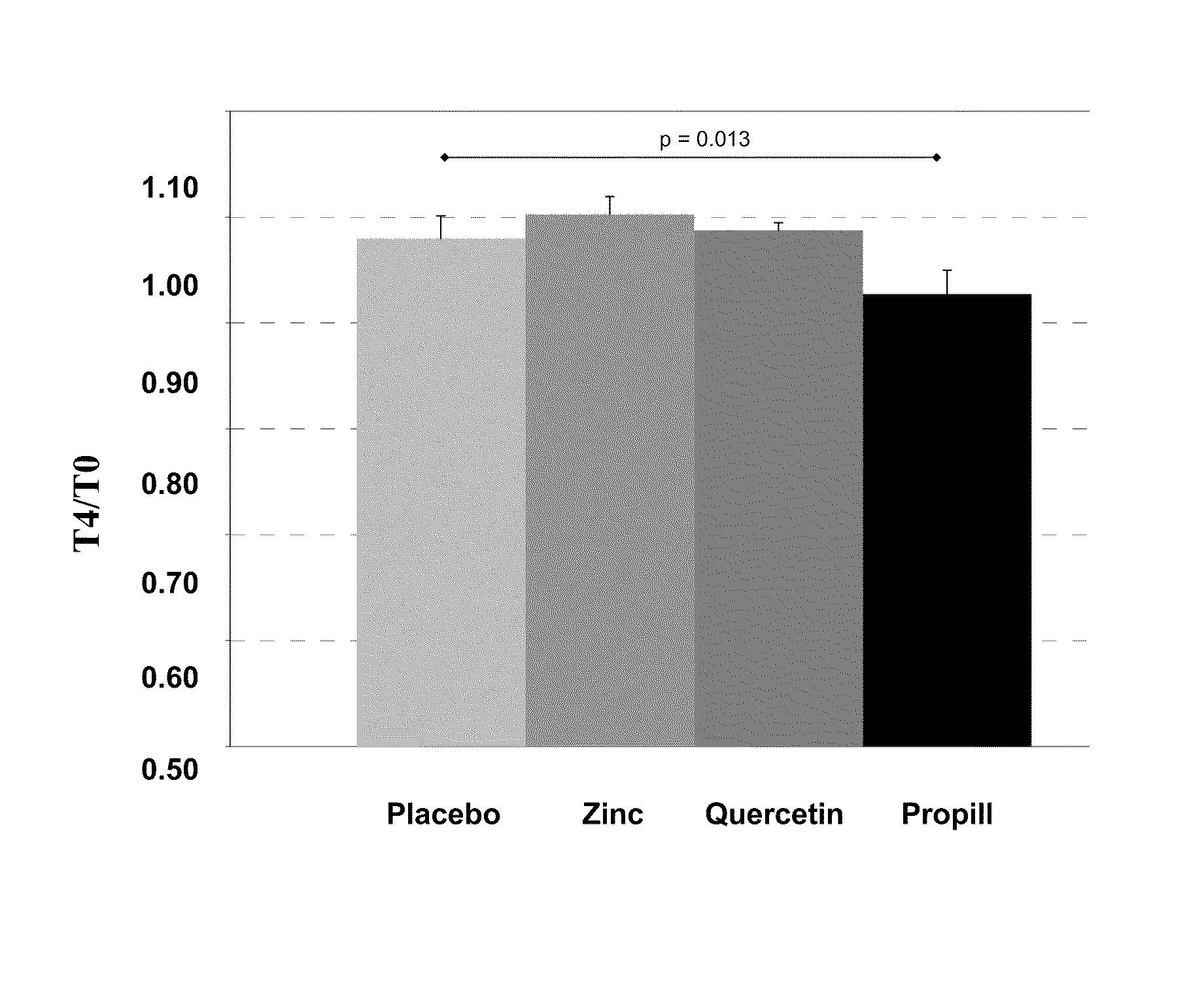
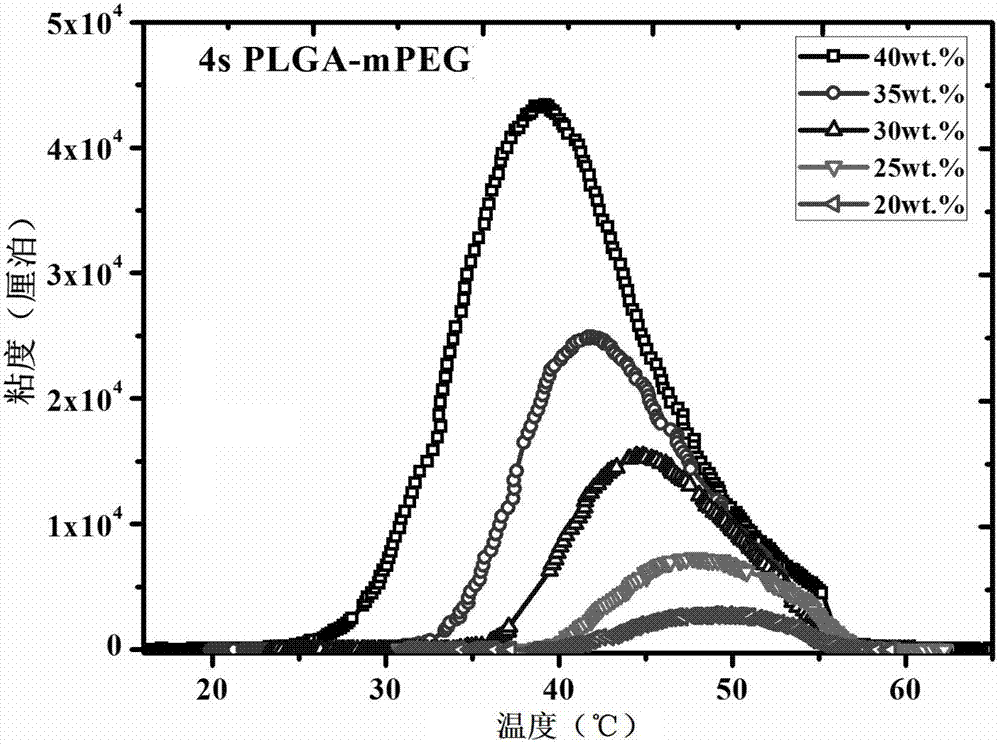
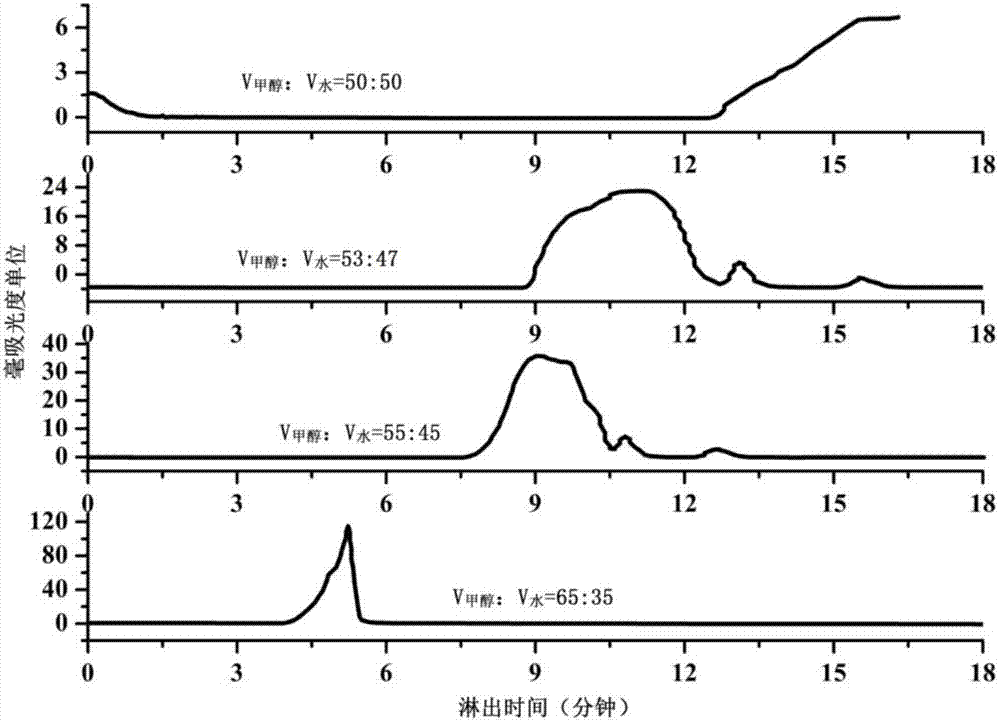
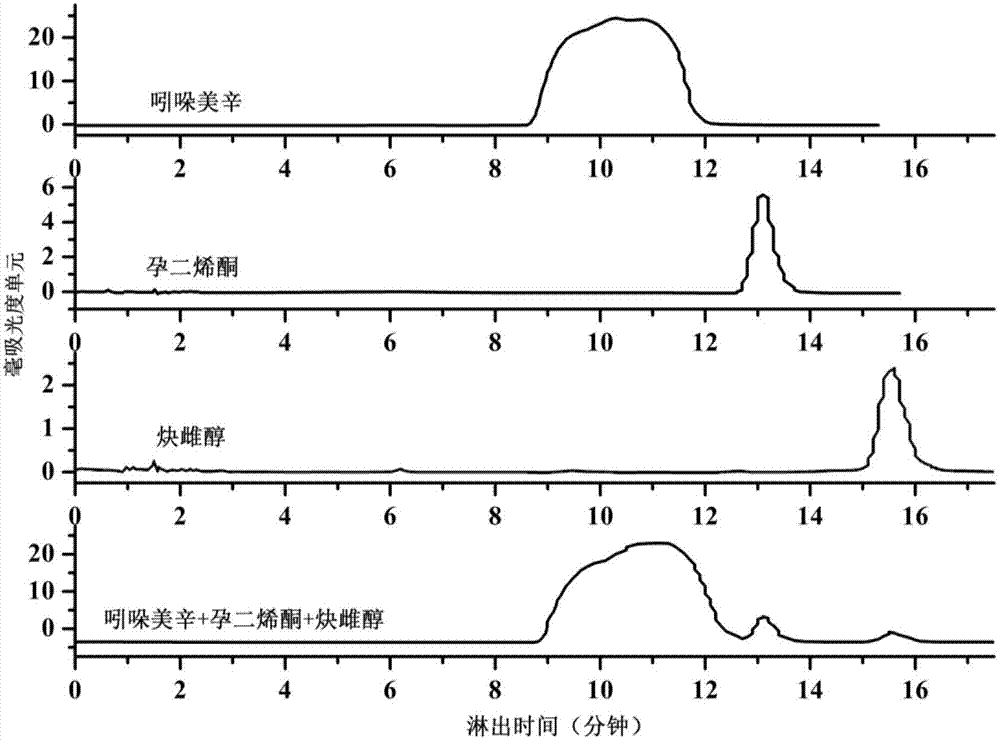
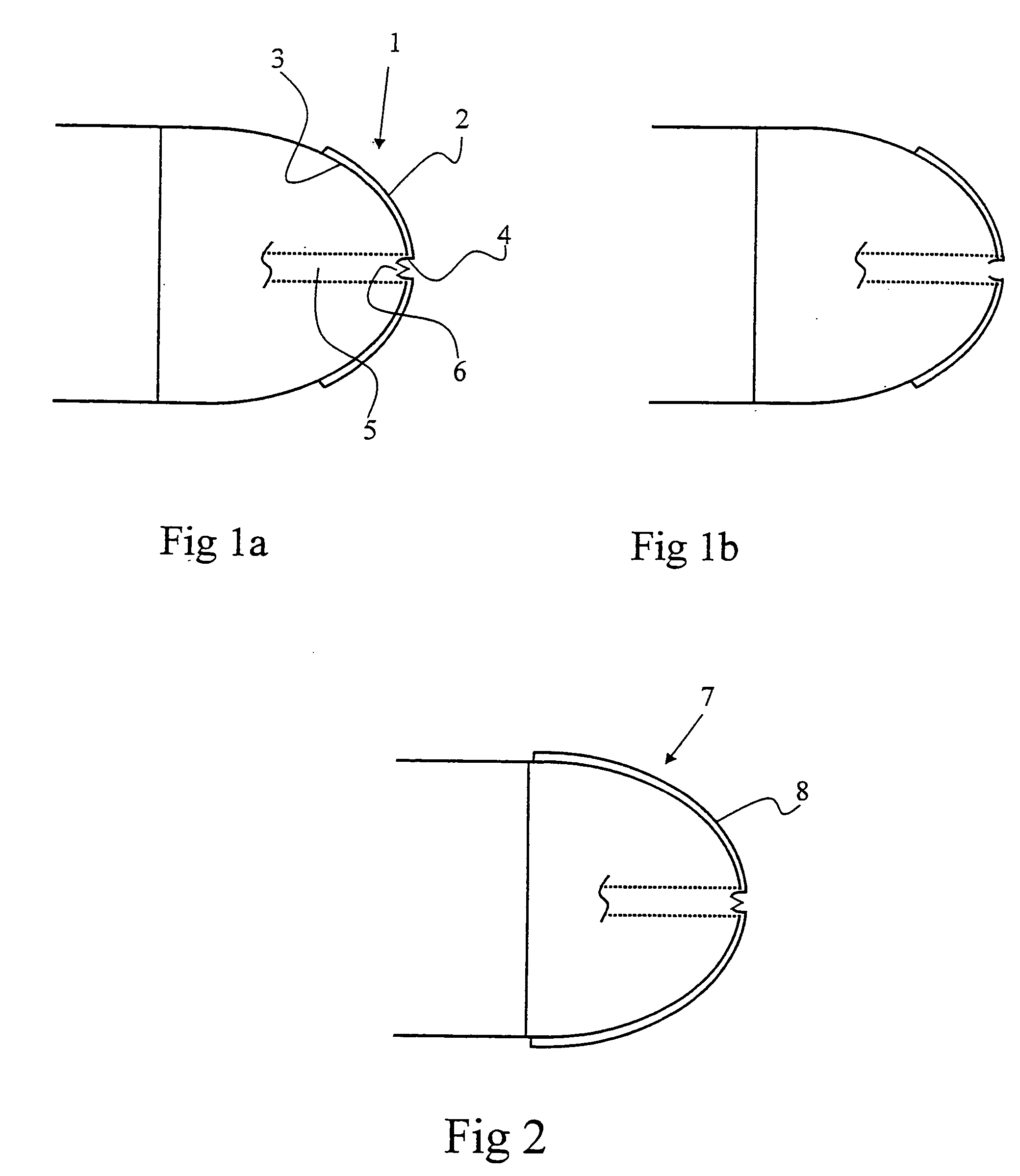
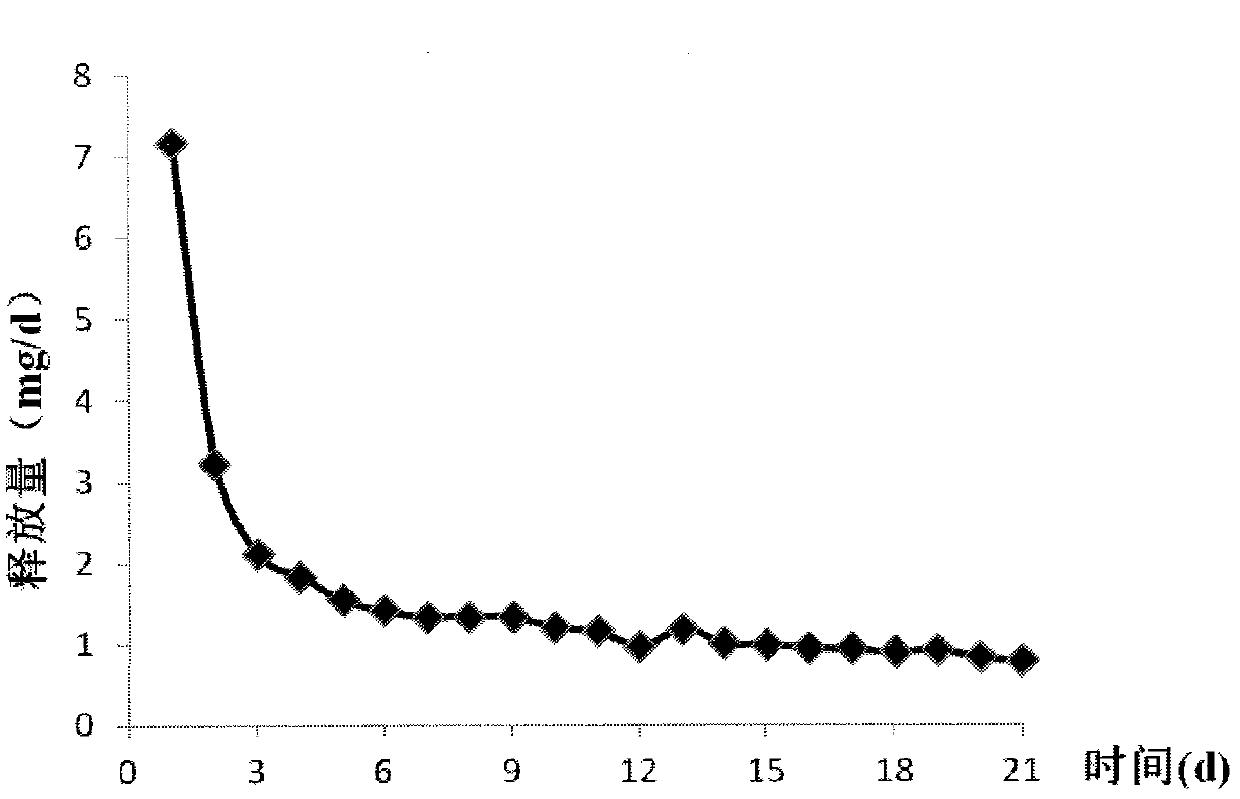
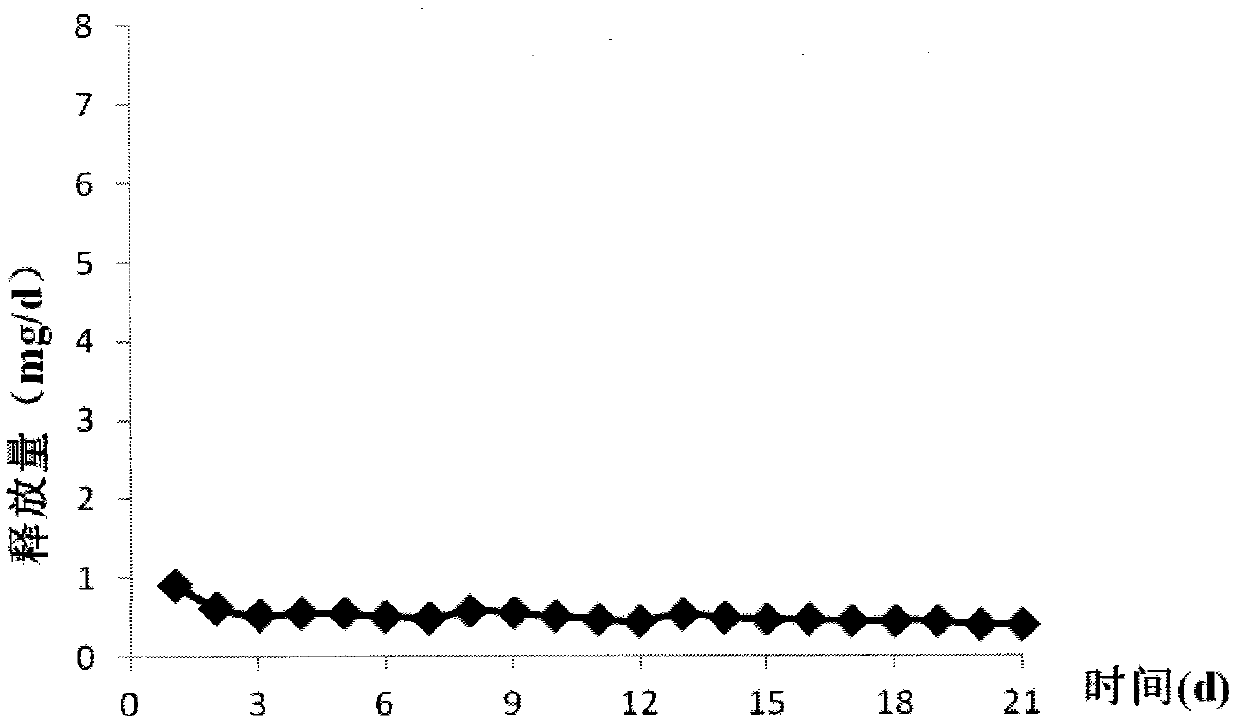
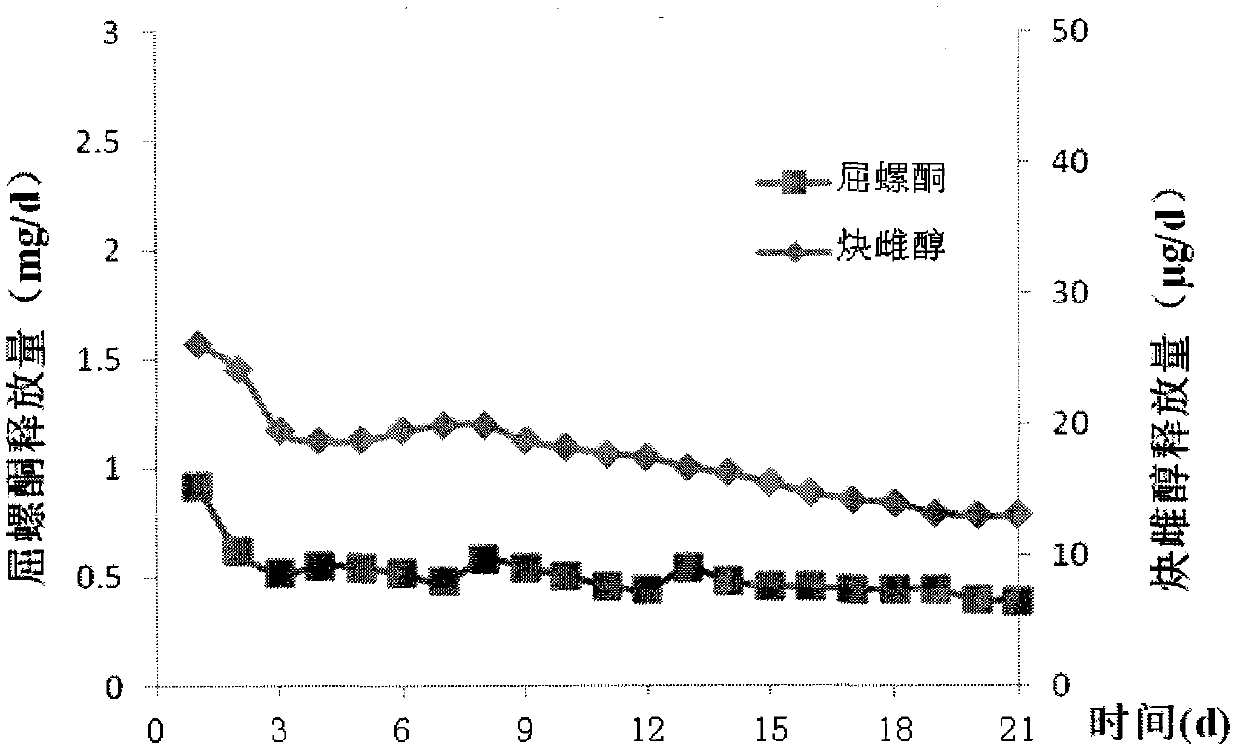
Patents
Literature
102 results about "Contraceptive drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Birth control pills (oral contraceptives) are prescription medications that prevent pregnancy. Three combinations of birth control pills that contain progestin and estrogen are 1) monophasic, 2) biphasic, and 3) triphasic. Birth control pills may also be prescribed to reduce menstrual cramps or prevent anemia.
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
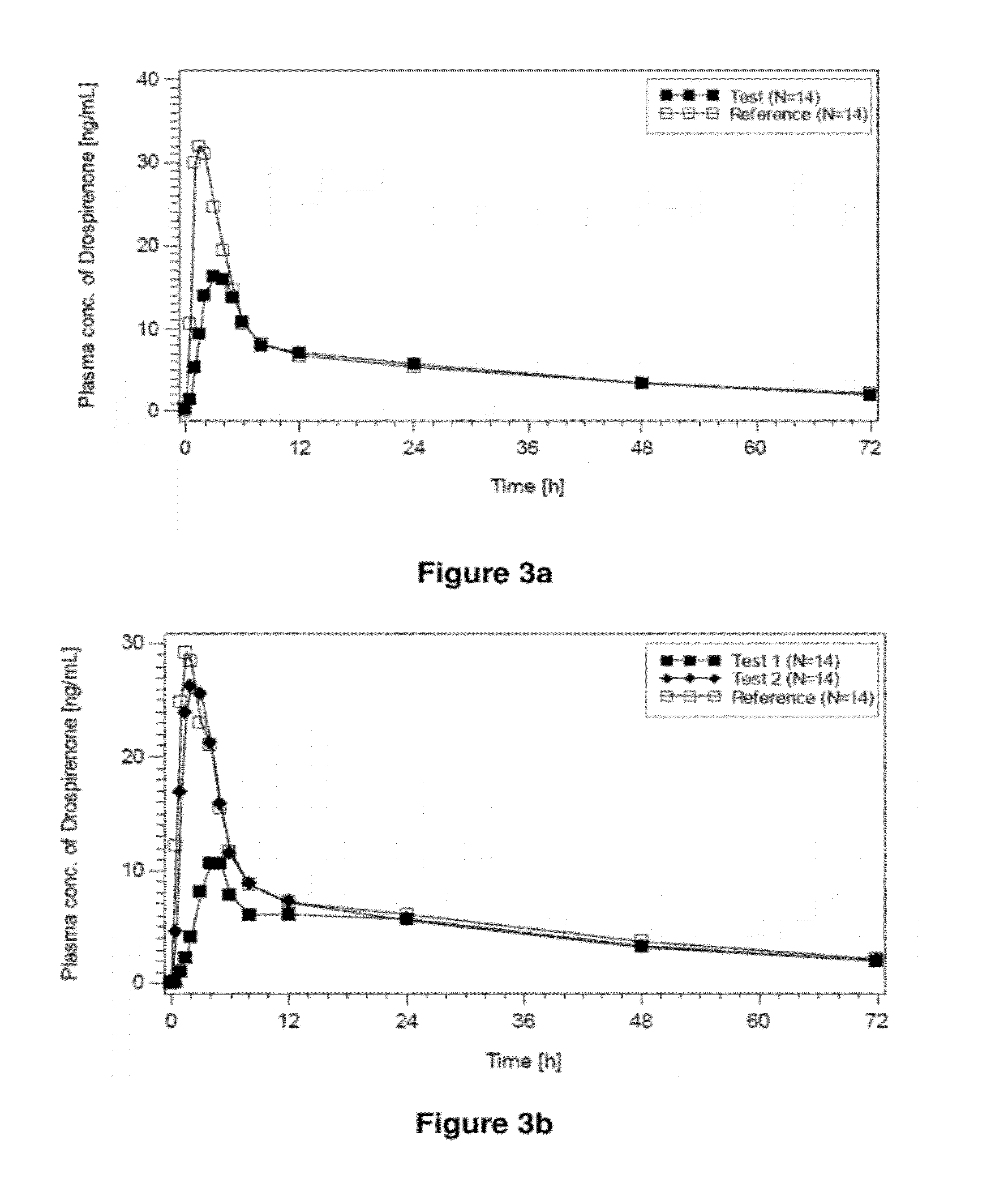
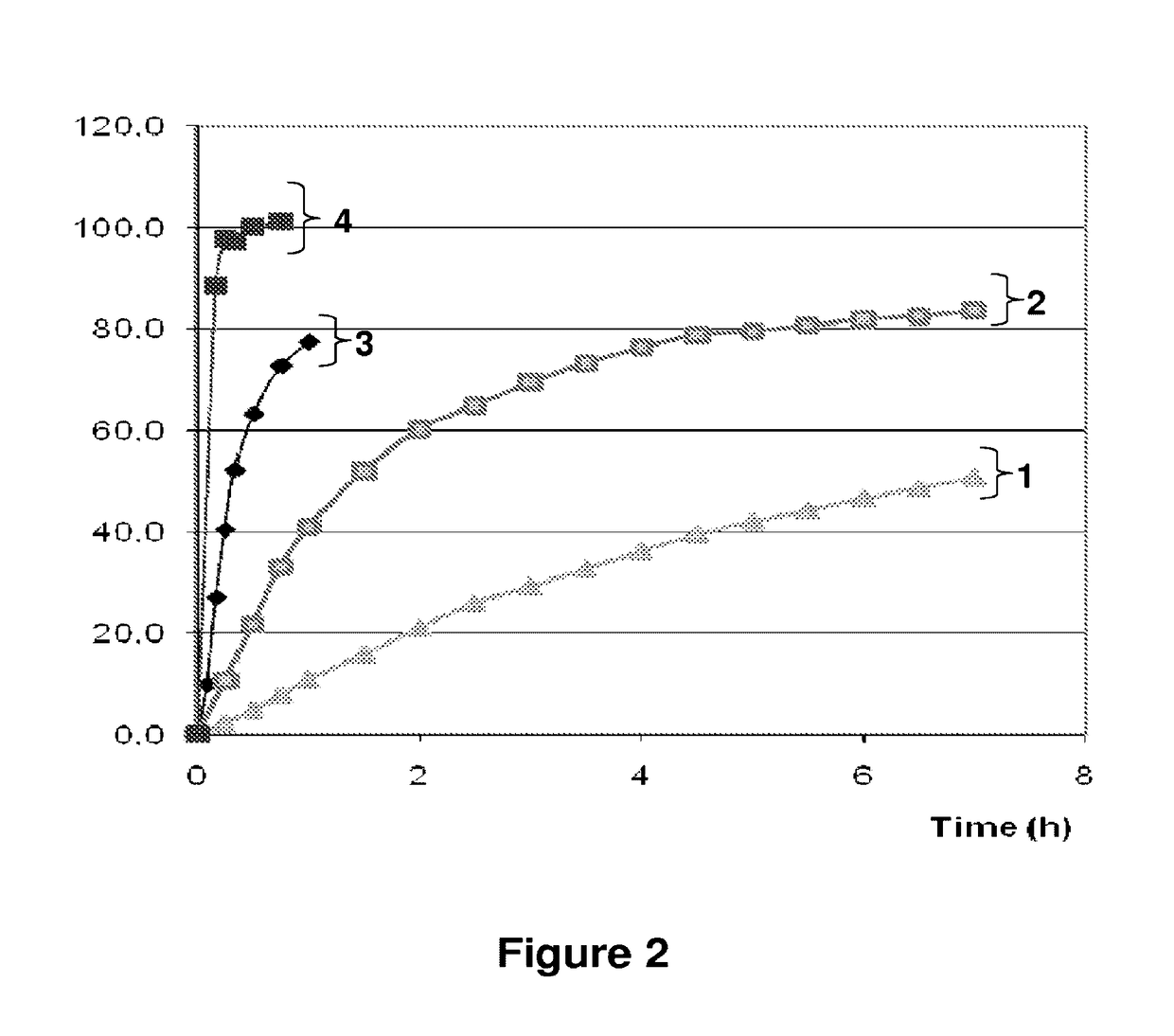
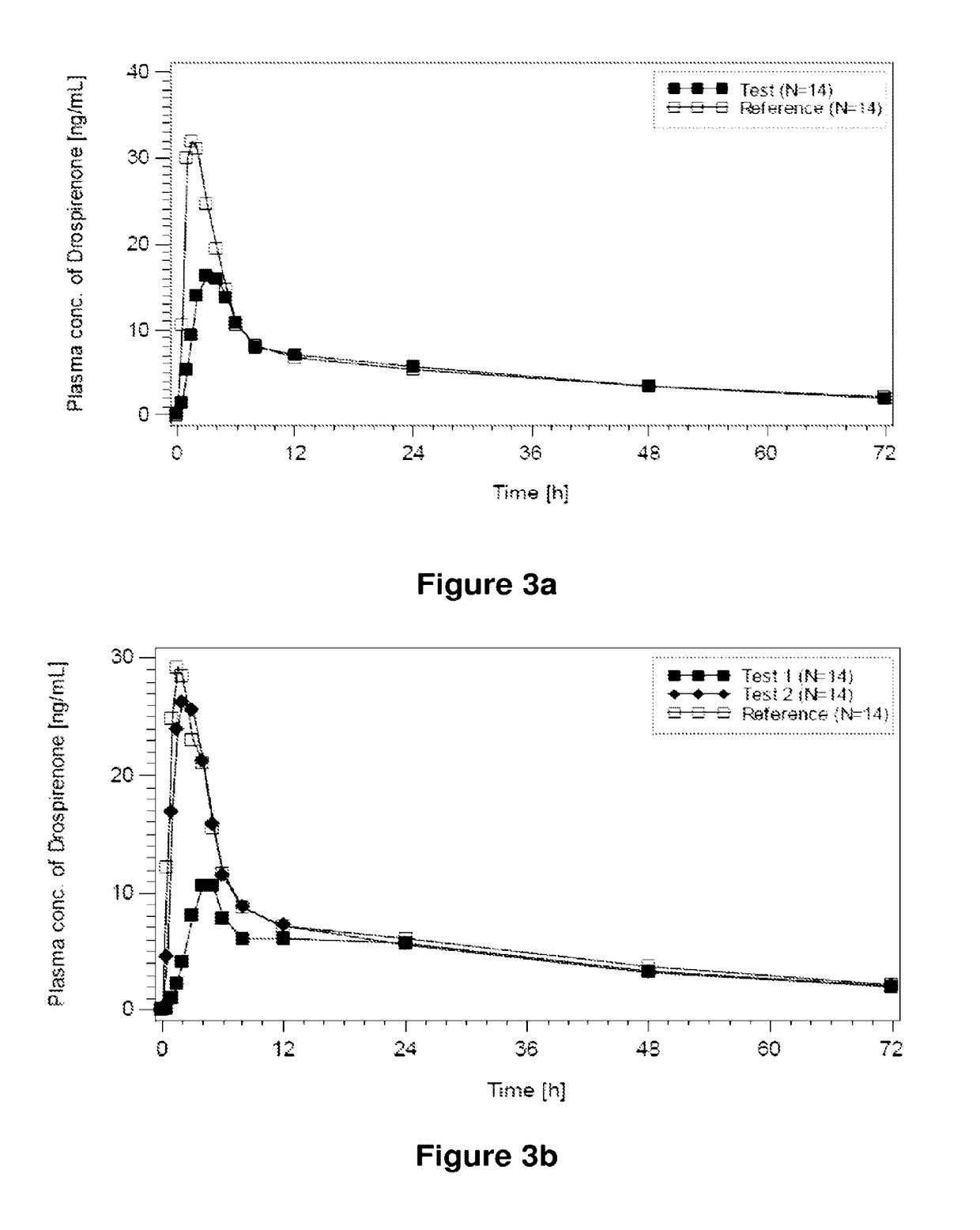
ActiveUS20120128733A1Inhibit ovulationBiocideOrganic active ingredientsDrospirenoneProgestogen-only contraception
The present invention relates to pharmaceutical compositions and kits comprising pharmaceutical compositions, and methods for administering pharmaceutical compositions comprising active contraceptive drugs in a patient. Specifically, the pharmaceutical compositions may comprise progestogen-only contraceptives (“POC”), such as Drospirenone.
Owner:LAB LEON FARMA
Composition for the treatment of oxidative stress
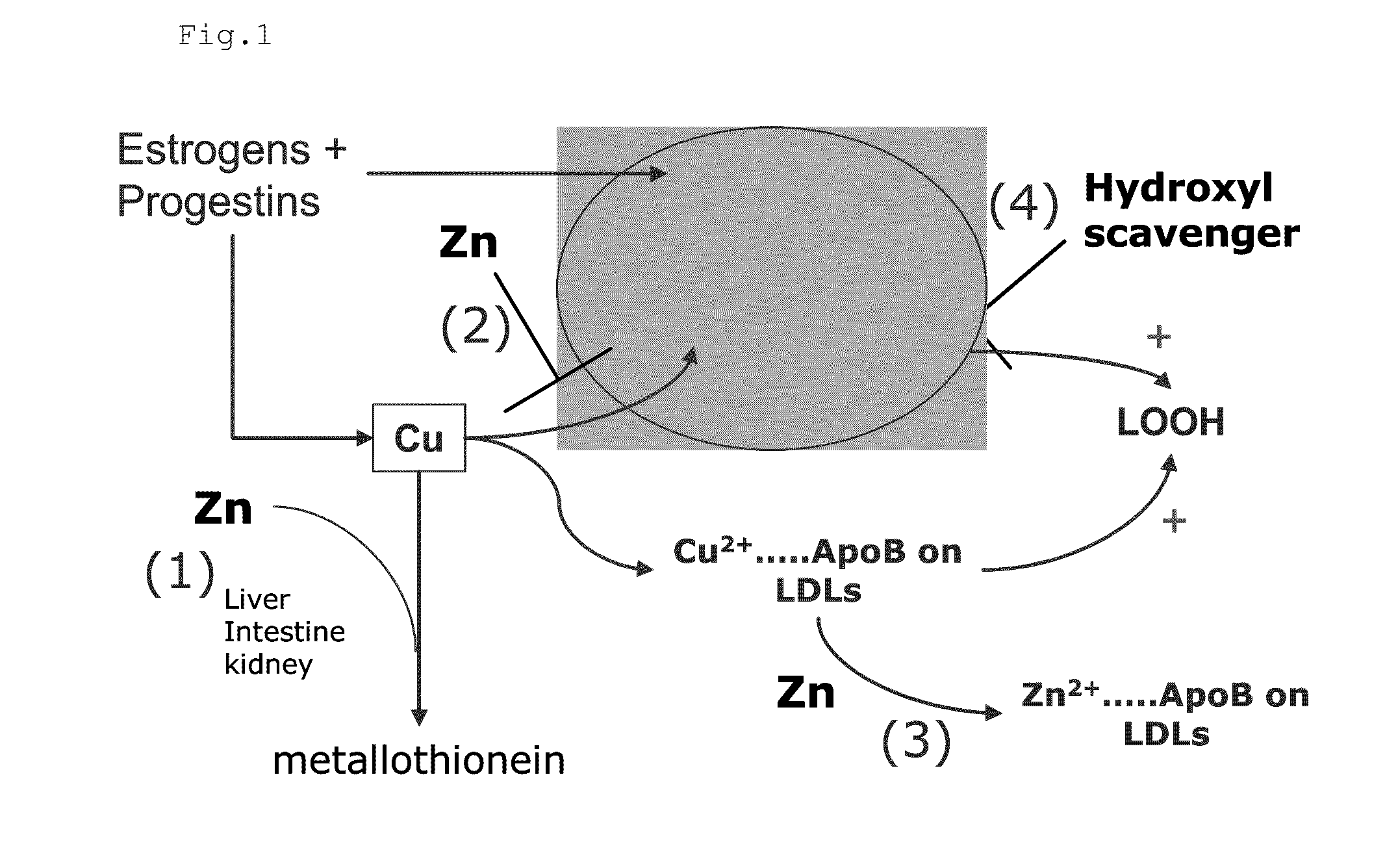
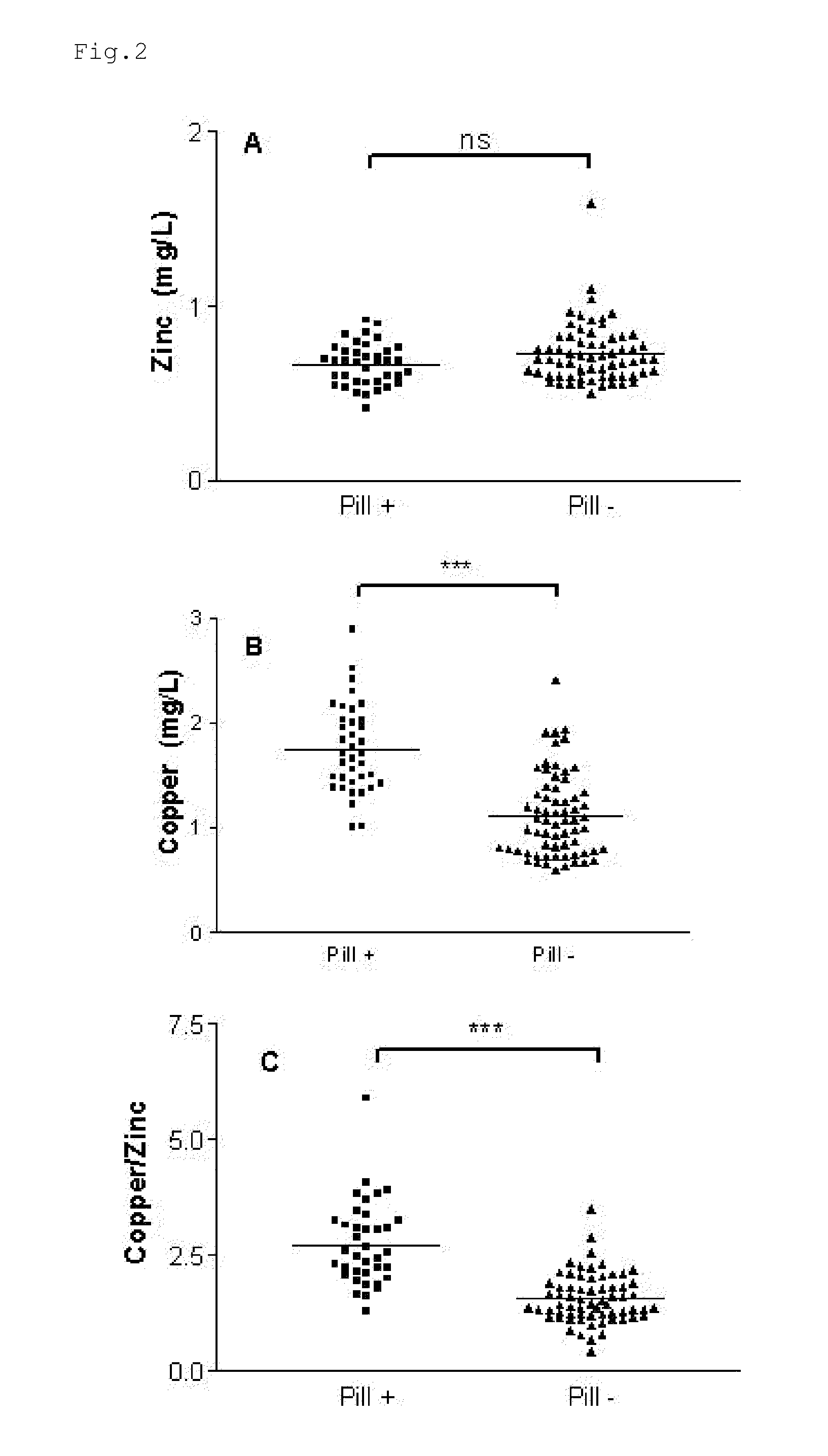
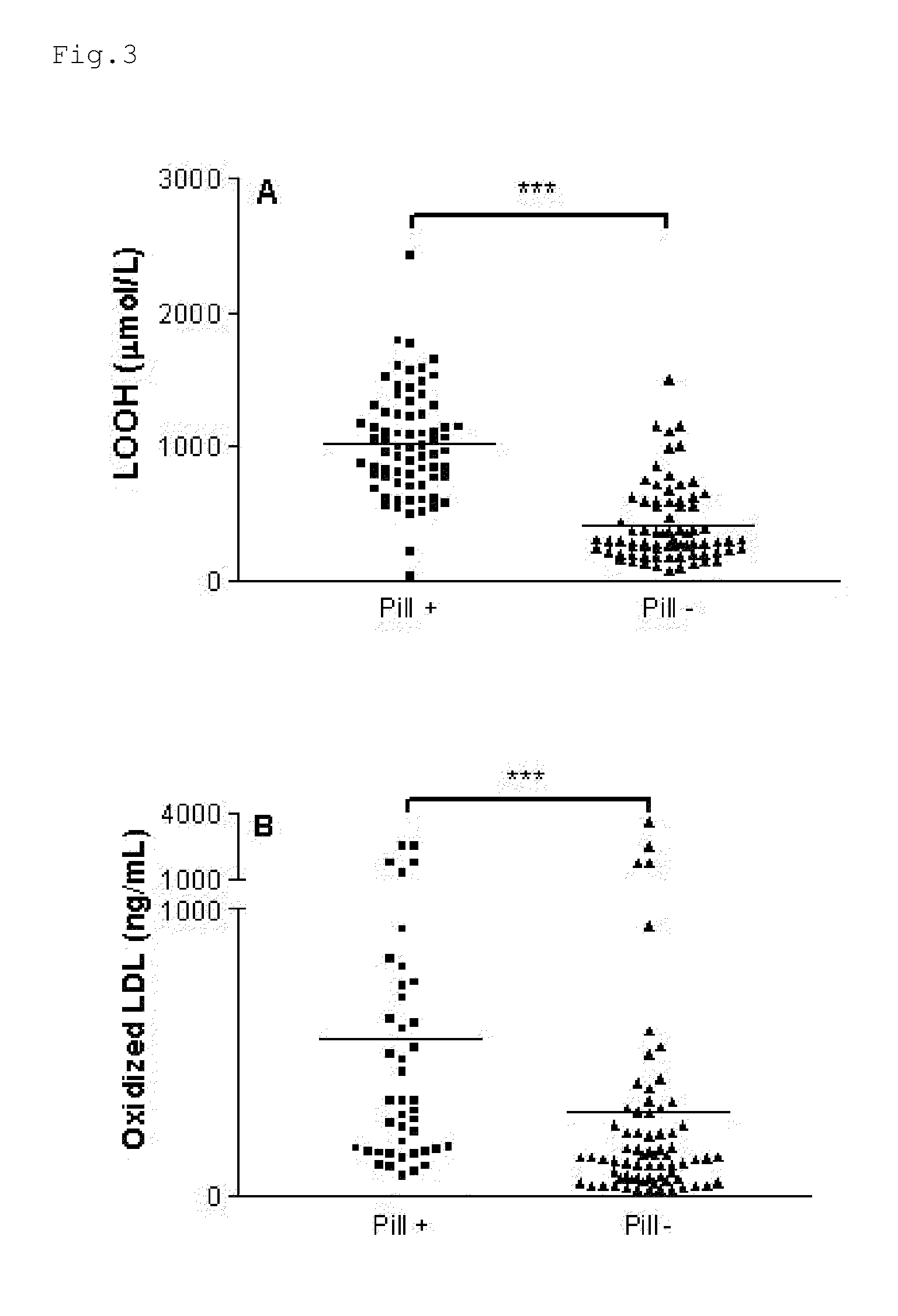
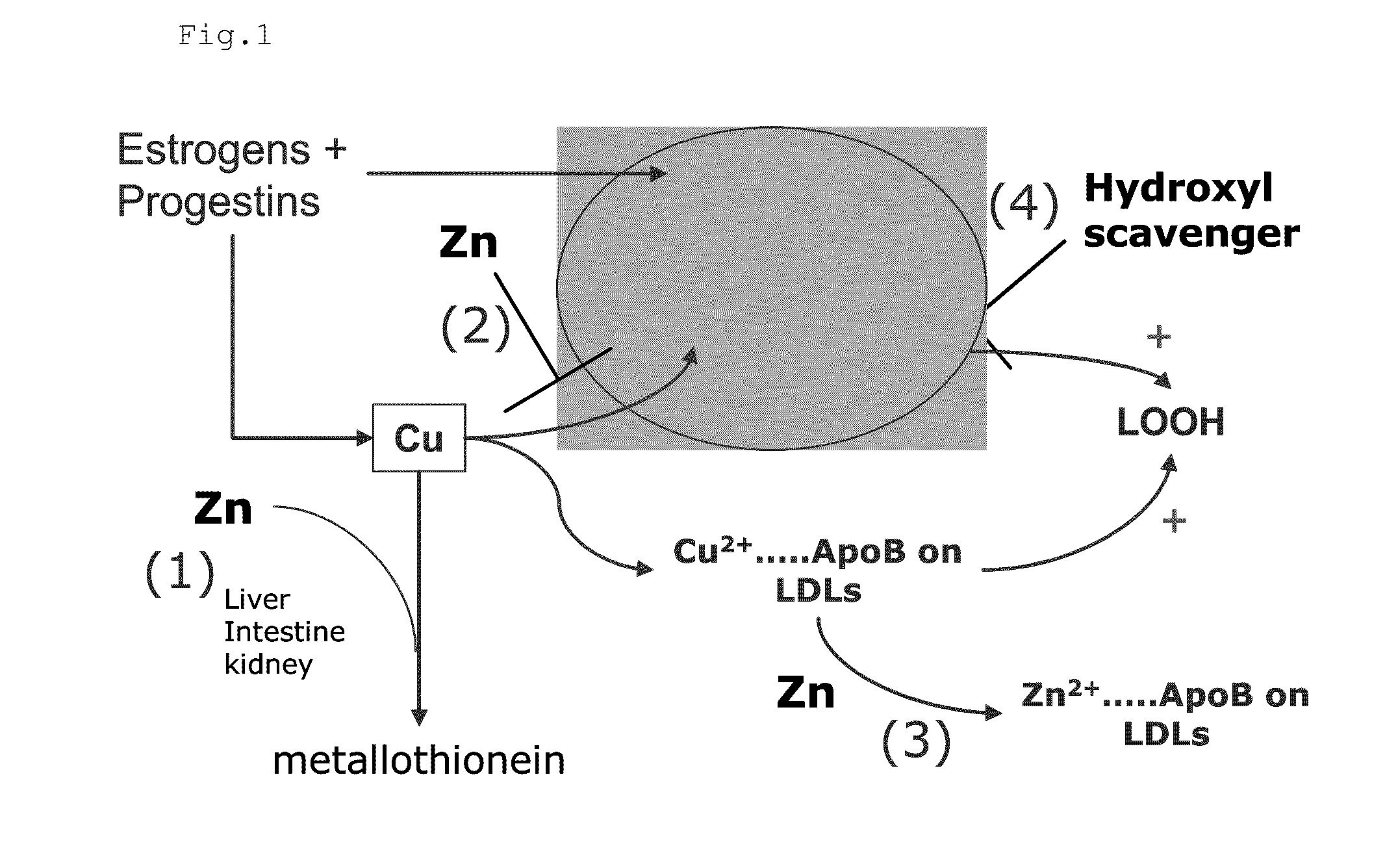
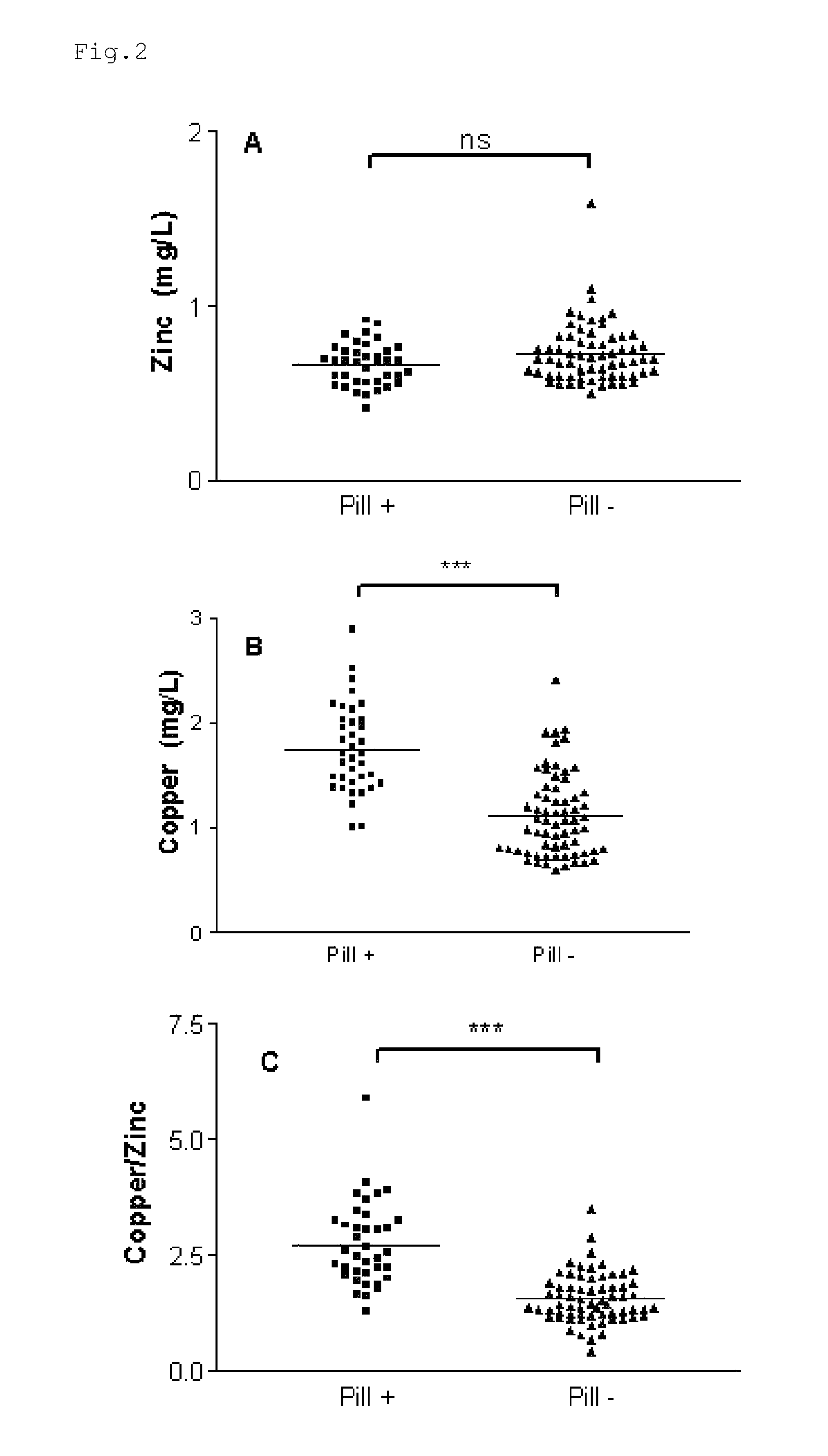
InactiveUS20120009276A1Preventing and or reducing increased lipid peroxidationEnhanced lipid peroxidationBiocideHeavy metal active ingredientsScavengerHormone replacement
This invention is based on the observed oxidative stress and increased risk on cardiovascular diseases in subjects with increased lipid peroxidation, in particular with women using oral contraceptives and in hormone replacement therapies. The invention provides compositions and combinations, particularly useful in preventing and or reducing the increased lipid peroxidation in subjects in need thereof. These compositions are based on the synergistic combination of zinc and / or a hydroxyl radical scavenger in reducing lipid peroxidation.
Owner:PROBIOX
Pharmaceutical composition containing a tetrahydrofolic acid
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Method of obtaining male contraception
ActiveUS20090250068A1Reduce and even eliminate riskSatisfactory blood circulationMale contraceptivesFallopian occludersVas deferensLower risk
There is provided a method for controlling a flow of fluid in the vas deferens in order to obtain a controlled male contraception. The method comprises gently constricting (i.e., without substantially hampering the blood circulation in the tissue wall) at least one portion of the tissue wall to influence the flow vas deferens, and stimulating the constricted wall portion to cause contraction of the wall portion to further influence the flow in the vas deferens. The method can be used for restricting or stopping the flow in the vas deferens, or for actively moving the fluid in the vas deferens, with a low risk of injuring the organ.
Owner:FORSELL PETER
Non-steroidal progesting
InactiveUS7388006B2Suitable for useBiocideOrganic chemistryPR - Progesterone receptorOral contraceptive drug
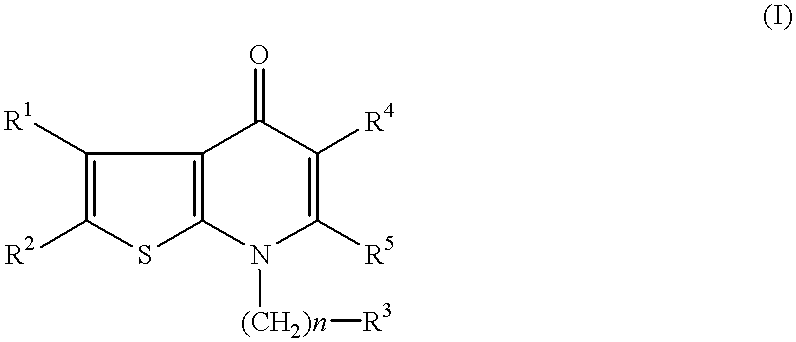
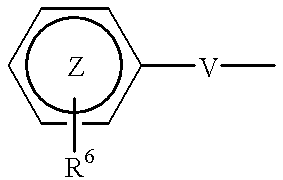
The present invention relates to non-steroidal progestins of the general formula (I)whereinR1 and R2 are independently of each other —H or —F,R3 is —CH3 or —CF3, andAr isor a pharmaceutically acceptable derivative or analogue thereof. These progestins are suitable for selectively modulating progesterone receptor mediated effects in different target tissues, particularly in uterine tissue versus breast tissue. Therefore, the progestins of the present invention, optionally in combination with estrogens, may be used for contraception (in particular in estrogen-free oral contraceptives), hormone replacement therapy and the treatment of gynecological disorders. The present invention furthermore relates to methods for selectively modulating progesterone receptor mediated effects in different target tissues or organs.
Owner:BAYER SCHERING PHARMA AG
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
ActiveUS9603860B2Inhibit ovulationPowder deliveryOrganic active ingredientsInitial treatmentPharmaceutical drug
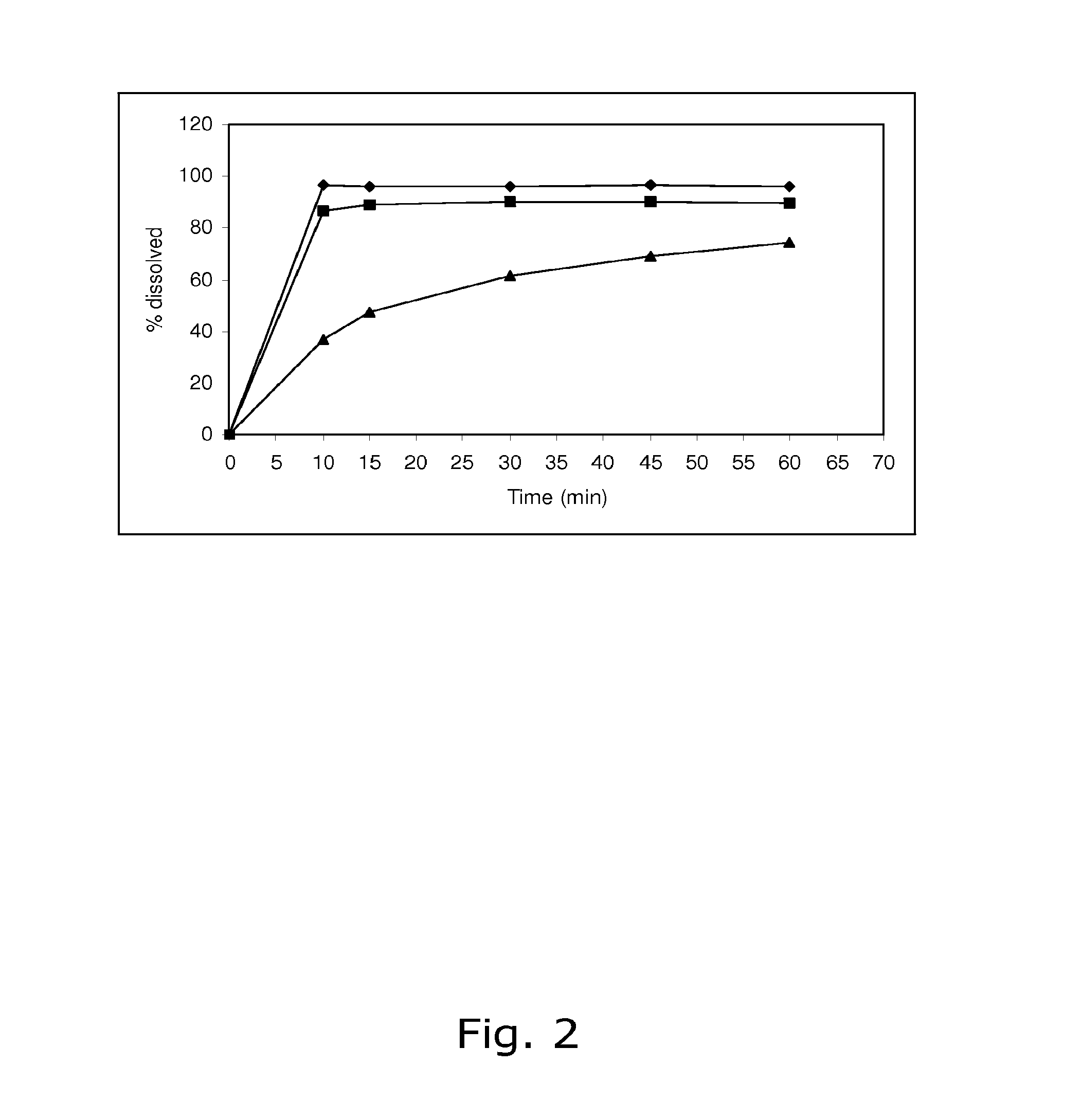
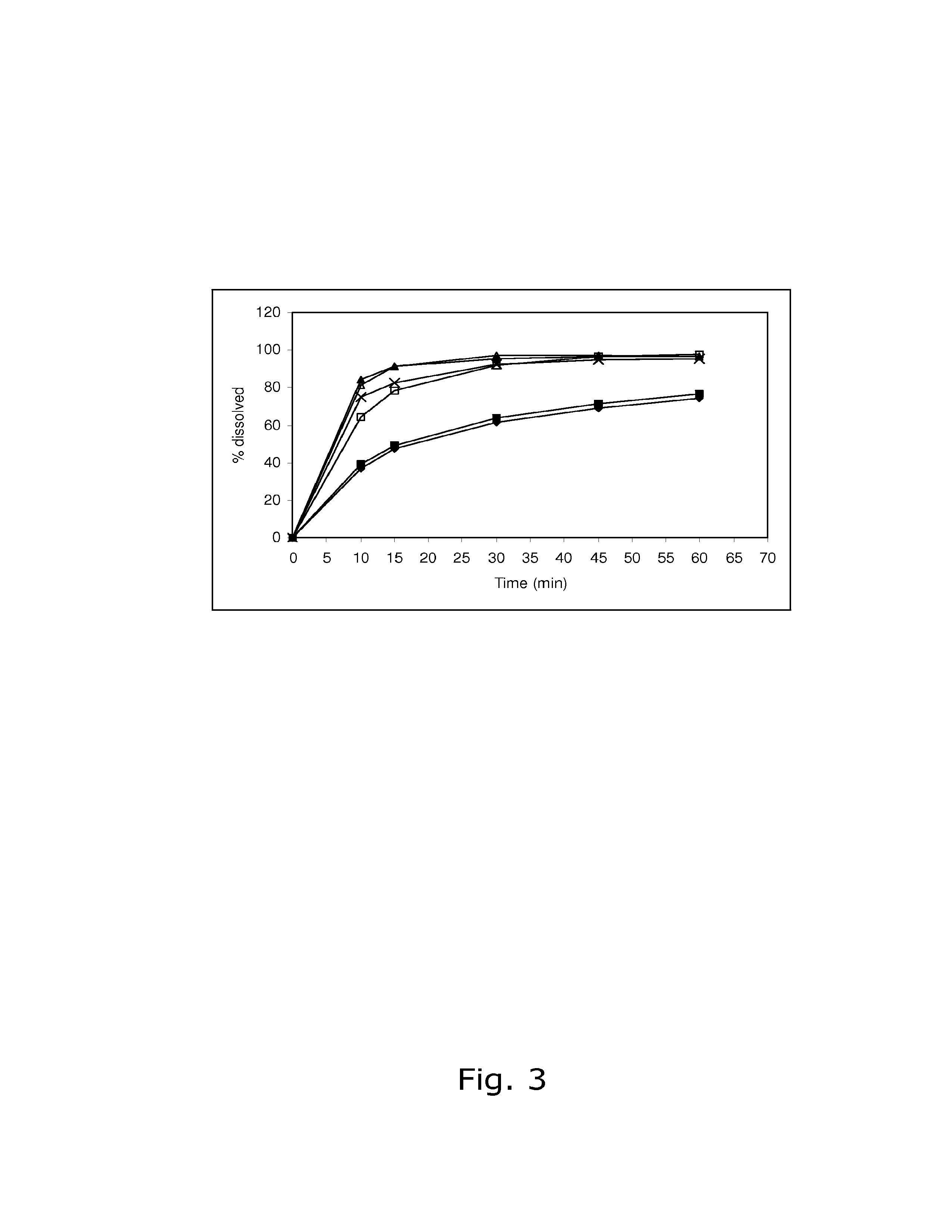
A pharmaceutical composition comprising an active contraceptive drug and one or more pharmaceutically-acceptable excipients. The pharmaceutical composition, when subjected to an in vitro dissolution test according to the USP XXIII Paddle Method, results in no more than 50% of said active drug initially present being dissolved within 30 minutes, and at least 50% of the active drug being dissolved in a time range from about 3 hours to about 4 hours. The pharmaceutical composition is administered daily to a patient having a BMI of about 25 kg / m2 or more for at least a portion of a treatment cycle. The pharmaceutical composition does not cause a number of days of bleeding events in the patient exceeding an average of 15% per treatment cycle in consecutive treatment cycles of administration after an initial treatment cycle of administration.
Owner:LAB LEON FARMA
Condensed-ring thiophene derivatives, their production and use
A gonadotropin-releasing hormone antagonistic composition, which comprises an optionally substituted condensed-bicyclic compound consisting of a homo or hetero 5 to 7 membered ring and a homo or hetero 5 to 7 membered ring is effective as a propylactic or therapeutic agent for the prevention or treatment of several hormone dependent diseases, for example, a sex hormone dependent cancer (e.g. prostatic cancer, cancer of uterine cervix, breast cancer, pituitary adenoma), benign prostatic hypertrophy, myoma of the uterus, endometriosis, precocious puberty, amenorrhea, premenstrual syndrome, polycystic ovary syndrome and acne vulgaris; is effective as a fertility controlling agent in both sexes (e.g. a pregnancy controlling agent and a menstrual cycle controlling agent); can be used as a contraceptive of male or female, as an ovulation-inducing agent of female; can be used as an infertility treating agent by using a rebound effect owing to a stoppage of administration thereof; is useful as modulating estrous cycles in animals in the field of animal husbandry, as an agent fro improving the quality of edible meat or promoting the growth of animals; is useful as an agent of spawning promotion in fish.
Owner:TAKEDA PHARMA CO LTD
Combination progestin oral contraceptive regimen
InactiveUS20010020015A1Reducing breakthrough bleedingBiocideOrganic active ingredientsRegimenPhysiology
An oral contraception regimen which comprises sequentially administering two or more progestational agents exhibiting different effects on the human endometrium in combination with an estrogen. The invention is also directed to an extended use oral contraception regimen comprising the sequential administration of two or more progestational agents in combination with an estrogen.
Owner:ORTHO MCNEIL PHARM INC
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
This invention relates to a method of preventing pregnancy and treating PMS including PMDD. More particularly, the invention relates to a method, which involves administering one of several combination oral contraceptive regimens in combination with an antidepressant and a kit containing the same.
Owner:TEVA WOMENS HEALTH
Method for obtaining high-purity 17α-acetoxy-11β-(4-n,n-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione
The invention discloses a method of acquiring high-purity 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione, comprising the following steps: a) putting the acquired crude 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione in a proper solvent system to generate a pure 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; b) separating the 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; and c) carrying out recrystallization on the acquired solid. The compound used as a new oral emergency contraception can be taken in 120 h after unprotected sexual intercourse of women without a reduction of emergency contraception effect with the delay of the time of using drugs, and has good safety and survivability simultaneously.
Owner:SICHUAN UNIV
Extended cycle multiphasic oral contraceptive method
ActiveUS20070207945A1Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Bioerodible contraceptive implant and methods of use thereof
ActiveUS9980850B2Eliminate needOrganic active ingredientsFallopian occludersControlled releaseGynecology
A contraceptive drug delivery system is provided in the form of a controlled release, bioerodible pellet for subdermal implantation. The pellet is bioerodible, and provides for the sustained release of a contraceptive agent over an extended time period. Bioerosion products are water soluble, bioresorbed, or both, obviating the need for surgical removal of the implant. Methods of using the drug delivery system, including in female contraception, are also provided.
Owner:GESEA BIOSCI INC
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
InactiveUS20190269620A1Inhibit ovulationBiocideOrganic active ingredientsDrospirenoneIUD with progestogen
The present invention relates to pharmaceutical compositions and kits comprising pharmaceutical compositions, and methods for administering pharmaceutical compositions comprising active contraceptive drugs in a patient. Specifically, the pharmaceutical compositions may comprise progestogen-only contraceptives (“POC”), such as Drospirenone.
Owner:LAB LEON FARMA
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
InactiveUS20080064670A1Low ratePreventing pregnancyBiocideOrganic active ingredientsGynecologyObstetrics
This invention relates to a method of preventing pregnancy and treating PMS including PMDD. More particularly, the invention relates to a method, which involves administering one of several combination oral contraceptive regimens in combination with an antidepressant and a kit containing the same.
Owner:TEVA WOMENS HEALTH
Composition for the treatment of oxidative stress
InactiveUS8586629B2Enhanced lipid peroxidationReduce lipid peroxidationBiocideHeavy metal active ingredientsScavengerHormone replacement
Owner:PROBIOX
ESC42 protein, preparation method and use thereof
InactiveCN1840544APeptide/protein ingredientsImmunoglobulins against animals/humansGeneAntibacterial drug
The provided human epididymis specific express protein ESC42 with coded sequence can be prepared by chemical method or genetic engineering technique, and can be used to prepare antibacterial drug or new contraceptive.
Owner:李建远
Percutaneous contraceptive drugs delivery system and method
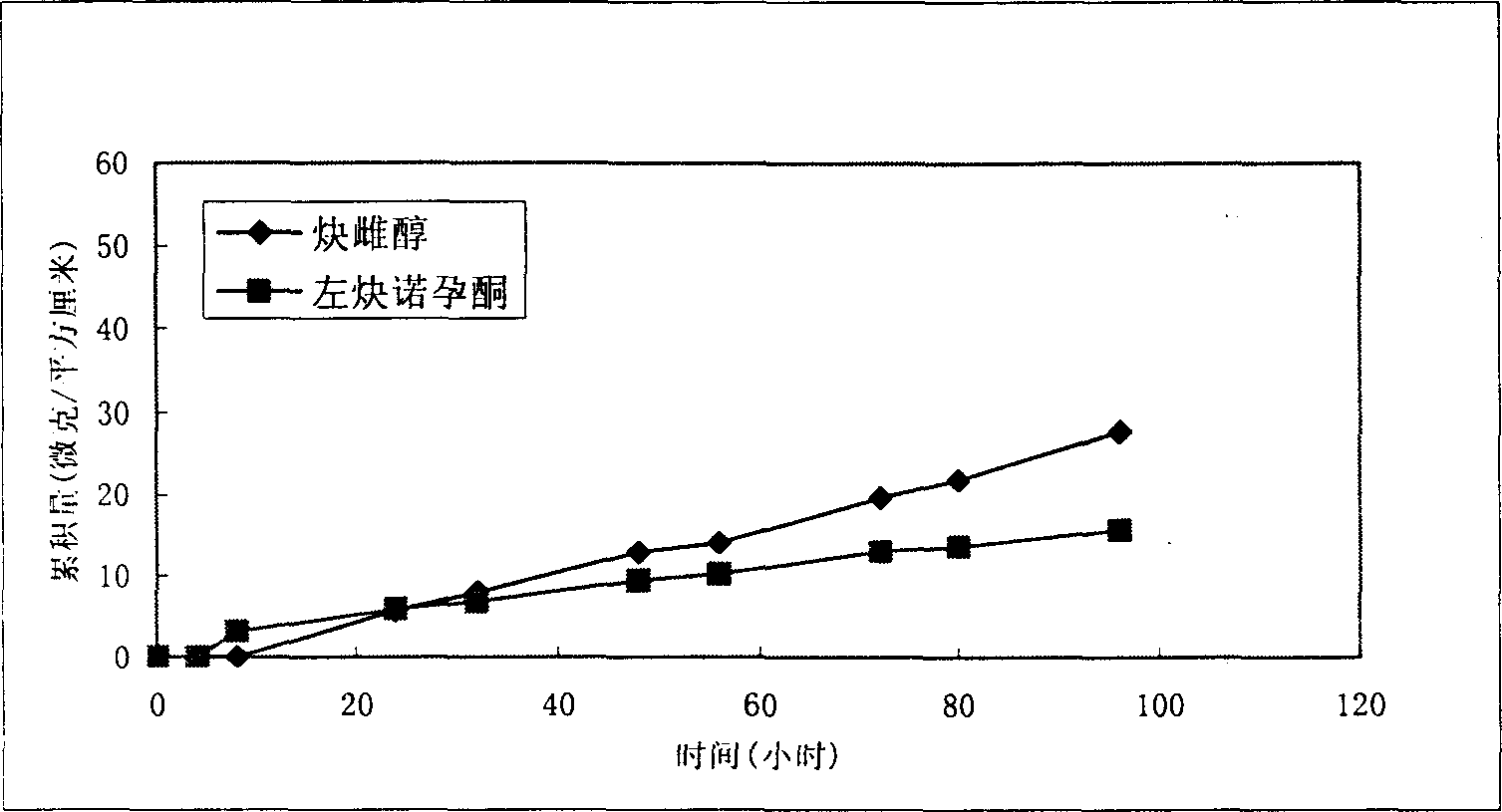
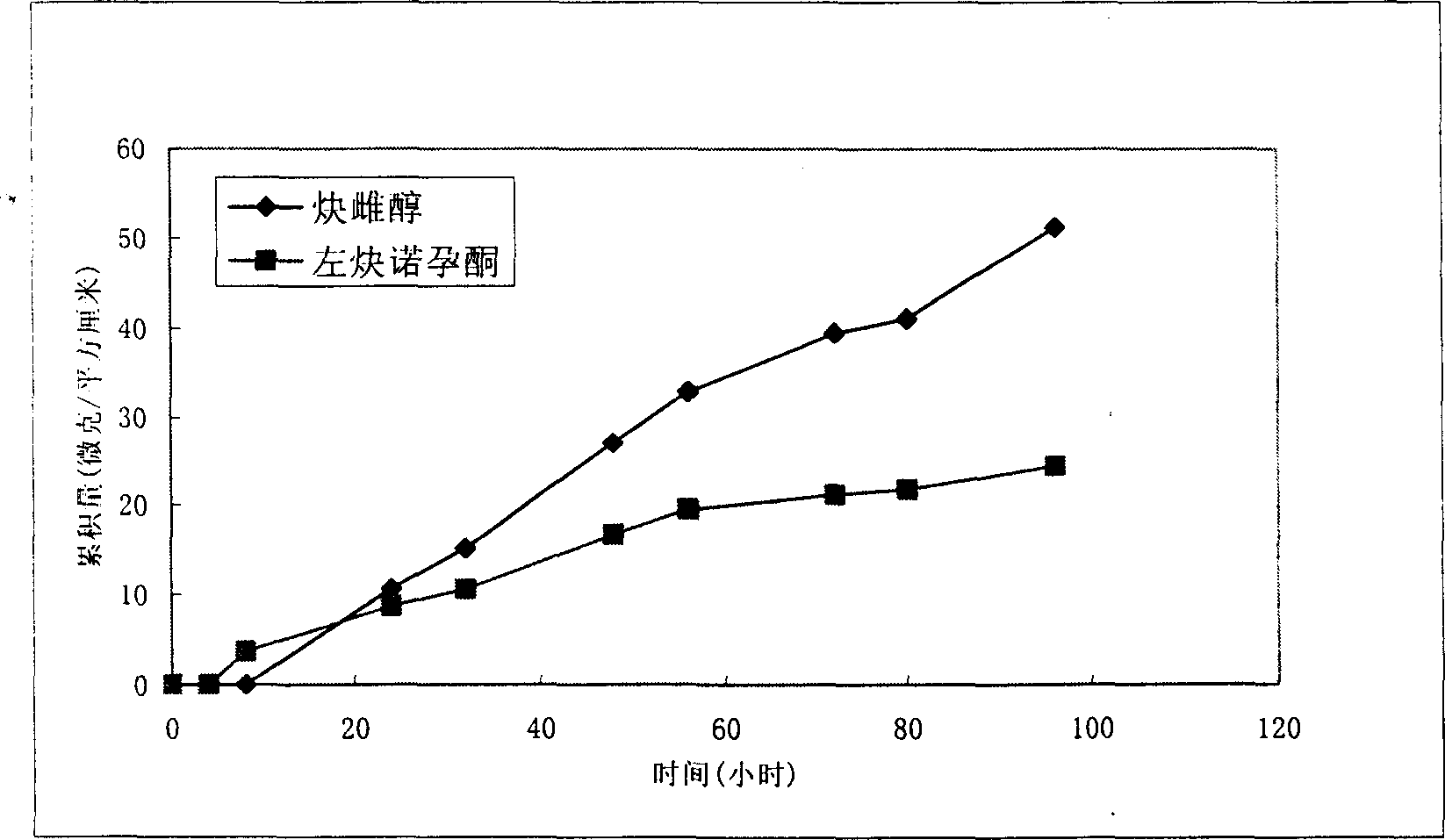
ActiveCN1633995AImprove skin penetration rateHigh hormone contentOrganic active ingredientsMedical devicesDiethylene glycolDelivery system
The invention provides a percutaneous contraceptive drugs delivery system and method, wherein the delivery system (TCDS) comprises a back lining layer, a viscous polymeric medicine storage layer adhered on the back lining layer, a strippable protective layer adhered on the viscous polymeric medicine storage layer, the viscous polymeric medicine storage layer comprises skin permeable accelerant composition, humectant, viscous polymer, active dosage of one or more steroid hormones, wherein the skin permeable accelerant composition is a mixture of dimethyl sulphoxide, 2-hydroxypropanoic aliphatic alcohol ester, diethylene glycol alkyl ether and oleinic acid by the initial weight ratio of 2 : 1 : 1 : 0.8 to 5 : 1 : 1 : 0.8. The invention can substantially improve steroid hormone transdermal speed and increase TCDS effectiveness and convenience.
Owner:RUNBIO BIOTECH CO LTD
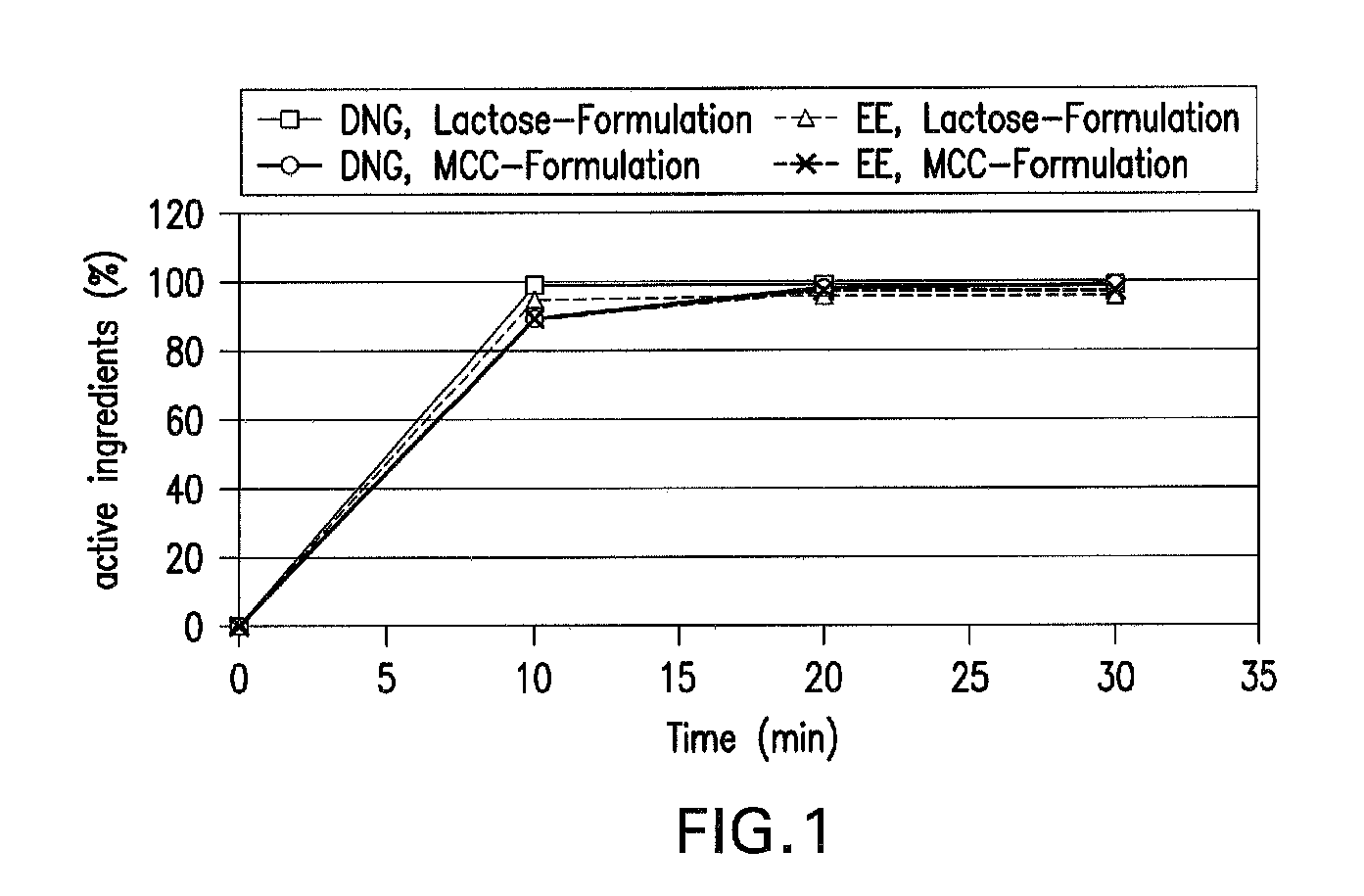
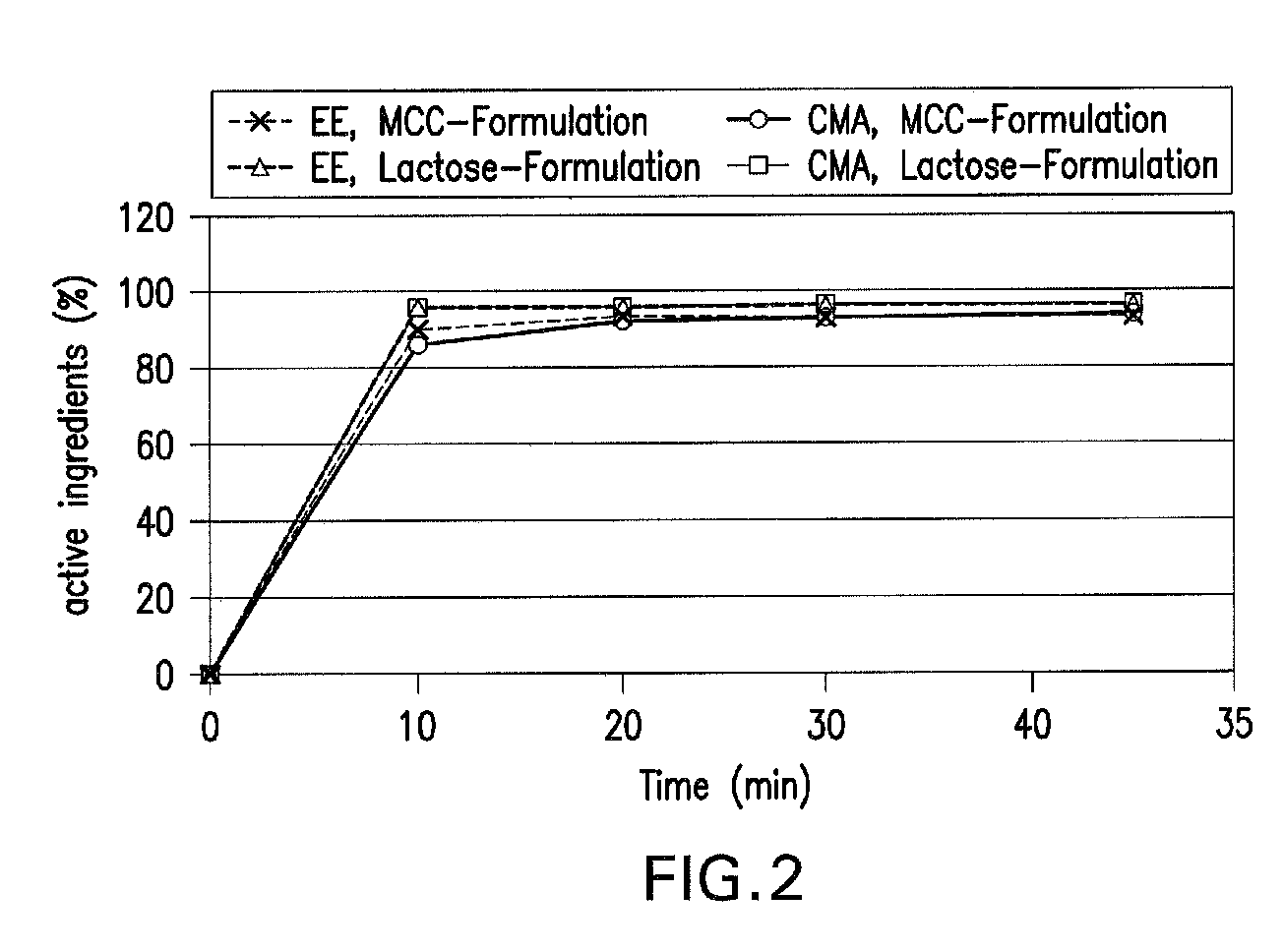
Oral contraceptive containing a gestagen and an estrogen combined with pharmaceutically acceptable auxiliary agents and/or excipients, but not containing lactose, and method of making same
InactiveUS20090117183A1Easy to solveLow costBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The method produces a lactose-free oral contraceptive composition containing a combination of a gestagen and an estrogen together with one or more pharmaceutically acceptable auxiliary agents and / or excipients. The contraceptive composition is a tablet, powder, or capsule that contains the gestagen and estrogen, filler material such as microcrystalline cellulose and a binder such as hydroxypropylcellulose, but no lactose. Preferably the gestagen is dienogest, chlormadinone acetate, or levonorgestrel and the estrogen is ethinylestradiol, 17β-estradiol, or estradiol valerate. A method is provided for improving the prophylaxis of lactose intolerance in women taking oral contraceptives. The oral contraceptive preparations for a standard 28-day cycle or for long-term use contain at least 21 daily dose units of the gestagen and the estrogen in a low-dosage but without lactose and at most 7 daily dose units containing no active ingredient or a placebo.
Owner:BAYER SCHERING PHARMA AG
Luteinizing hormone-releasing hormone (LHRH) antagonist derivative, preparation method of LHRH antagonist derivative and application of LHRH antagonist derivative
InactiveCN102675418AGood LHRH antagonist activityLow histamine releasing activityPeptide/protein ingredientsLuteinising hormone-releasing hormoneDiseaseSexual hormones
The invention belongs to the field of medicine and chemistry and particularly relates to a luteinizing hormone-releasing hormone (LHRH) antagonist derivative which is represented as formula (I), a preparation method of the LHRH antagonist derivative and application for preparation of drugs curing related sex hormone diseases or contraceptives. The invention also relates to a stereoisomeride of the LHRH antagonist derivative which is represented as the formula (I), a solvate of the LHRH antagonist derivative which is represented as the formula (I), or physio-toxicity-free salt of the LHRH antagonist derivative which is represented as the formula (I) and a pharmaceutical composition which contains compounds such as the LHRH antagonist derivative, the stereoisomeride, the solvate or the salt. An LHRH antagonist is modified with a water soluble group and a water soluble vitamin structure, the obtained LHRH antagonist derivative can maintain the activity of original antagonists, has low histamine-releasing activity and is beneficial to the clinic application and the water solubility is increased. The formula (I) is R-D-NAI-D-Cpa-D-Aaa3-Ser-Aaa5-Aaa6-Leu-Aaa8-Pro-D-Ala-B.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Temperature sensitive injectable drug-loading controlled release system
InactiveCN102755282APlay an anti-inflammatory rolePharmaceutical delivery mechanismPharmaceutical non-active ingredientsLactidePolythylene glycol
The invention discloses a temperature sensitive injectable drug-loading controlled release system, which comprises a star-shaped poly-lactic-co-glycolic acid-methoxy polyethylene glycol segmented copolymer and a simulated body fluid, and the content of the copolymer accounts for 15-40 weight by percent. The drug-loading controlled release system exists in a state of liquid which can freely flow in or below room temperature, is rapidly changed into oyster white physical crosslinking hydrogel which cannot flow at a human body temperature, and expresses a reversible sol- gel conversion behavior; the drug-loading controlled release system can uniformly disperse a loaded drug, and exists in a state of liquid which can freely flow in or below room temperature and gel which cannot freely flow at the body temperature; as a vagina drug-loading controlled release system, the temperature sensitive injectable drug-loading controlled release system has the characteristics that contraceptive compound estrogen, progestogen and an anti-inflammation drug are loaded into a copolymer hydrogel carrier by adopting a solution blending method, and the prepared drug-loading system serves as a birth control spraying agent; the drug-loading system is sprayed into vagina after women menses, and is shaped into mesh gel and attached into a wall, and the drug is slowly and controllably released and have an effect of birth control.
Owner:HUAZHONG UNIV OF SCI & TECH
Topical pharmaceutical composition comprising tenofovir, an antibacterial agent and,optonally ciclopirox
The present invention relates in general to a topical pharmaceutical composition comprising an antiretroviral agent in combination with a bactericidal agent and optionally an antifungal agent, particularly for use as a contraceptive.
Owner:CIPLA LTD
Prescription technique for improving stability of desogestrel
InactiveCN103271928AImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDesogestrelVitamin C
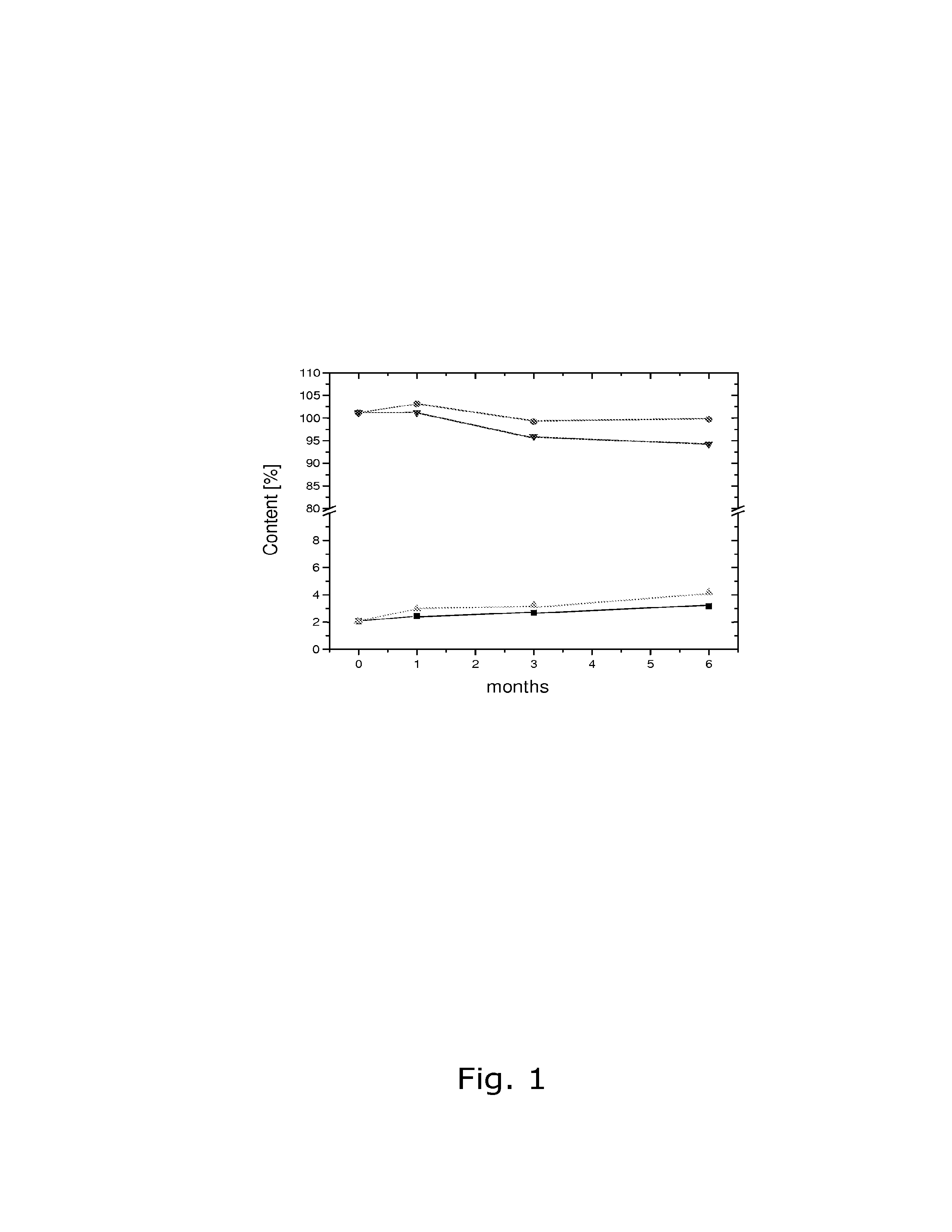
The invention discloses a prescription technique for improving the stability of desogestrel. According to the prescription technique, active medicines of the prescription are dissolved in a solvent mixture of which the weight is 5-200 times the weight of the active medicine; the active medicines are desogestrel and ethinylestradiol; the solvent mixture is chloroform or other solvent mixtures; and the active medicines are completely dissolved in the solvent mixture, and then an antioxygen of which the amount is 2-10 times the amount of the active medicines is added; the antioxygen including vitamin C or vitamin E is dissolved, so that the active medicines and the antioxygen are a solution at molecular distribution state, and each active medicine molecule is surrounded and protected by a plurality of antioxygen molecules. The prescription technique disclosed by the invention improves the stability of main medicines of the oral contraceptive and has the advantage that the shelf time of the oral contraceptive consisting of desogestrel and ethinylestradiol is as long as 24 months, etc.
Owner:NOVAST LABORATORIES (CHINA) LTD
Cannabimimetic lipid amides as useful medications
InactiveUS7161016B1High affinityHigh selectivityFatty acid chemical modificationOrganic compound preparationCancer and nauseaMetabolic stability
Novel analogs of arachidonylethanolamide are presented which have higher affinities for the cannabinoid CB1 and / or CB2 receptor sites. Further, most of the analogs exhibit greater metabolic stability than arachidonylethanolamide. The improved receptor affinity and selectivity and / or greater metabolic stability make these analogs therapeutically useful as medications for relief of pain caused by cancer and nausea caused by chemotherapy, as well as for peripheral pain. The compounds may also be useful as oral and topical contraceptives, in suppression of the immune system, enhancement of appetite and in treatment of psychomotor disorders, multiple sclerosis and hypertension.
Owner:UNIV OF CONNECTICUT
Non-mammalian GnRH analogs and uses thereof in regulation of fertility and pregnancy
InactiveUS20050054576A1Reduce and eliminate sperm productionDisable motilityPeptide/protein ingredientsGenetic material ingredientsDiseasePregnancy
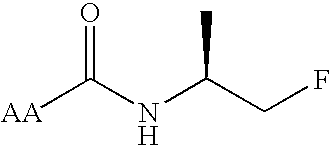
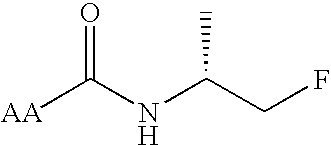
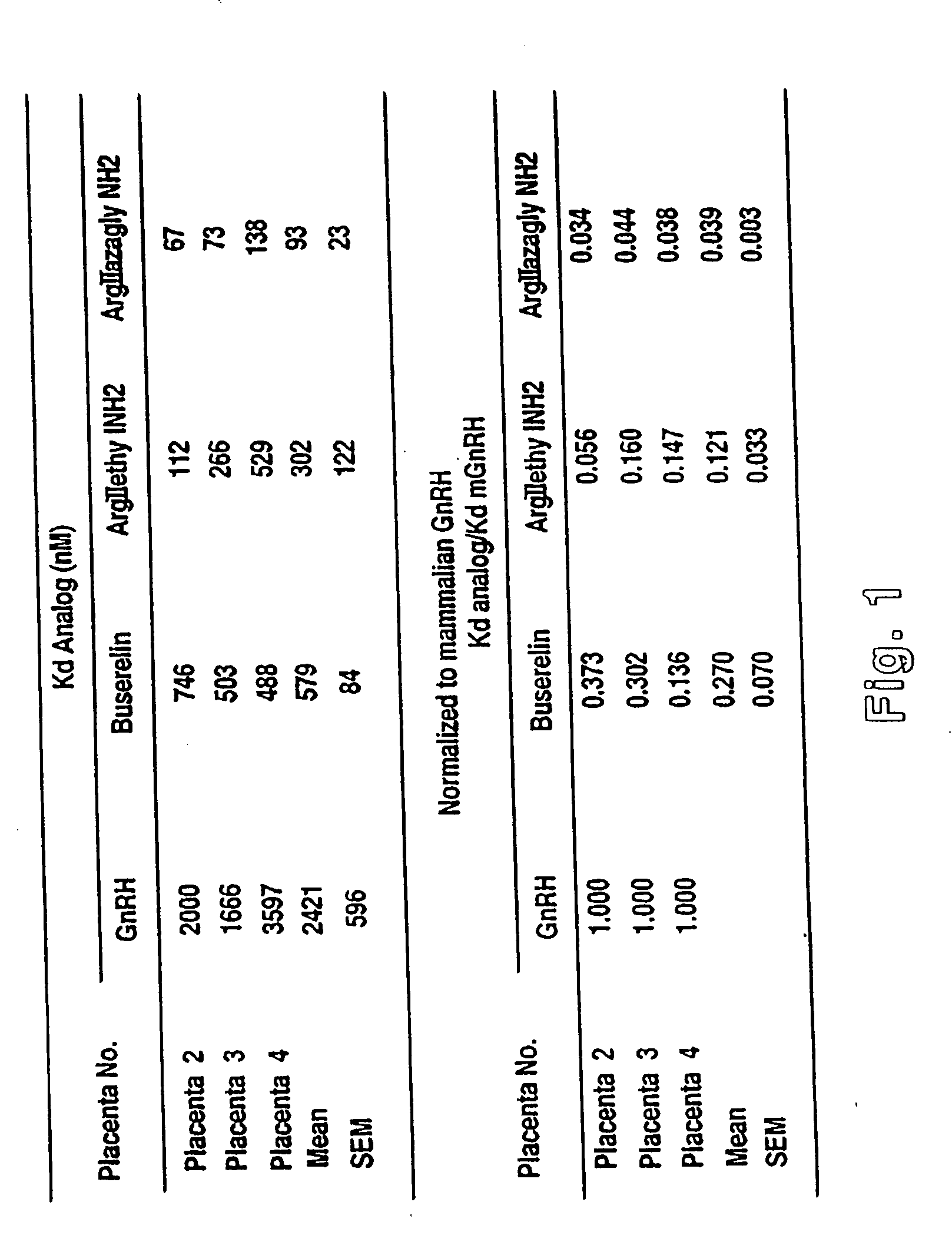
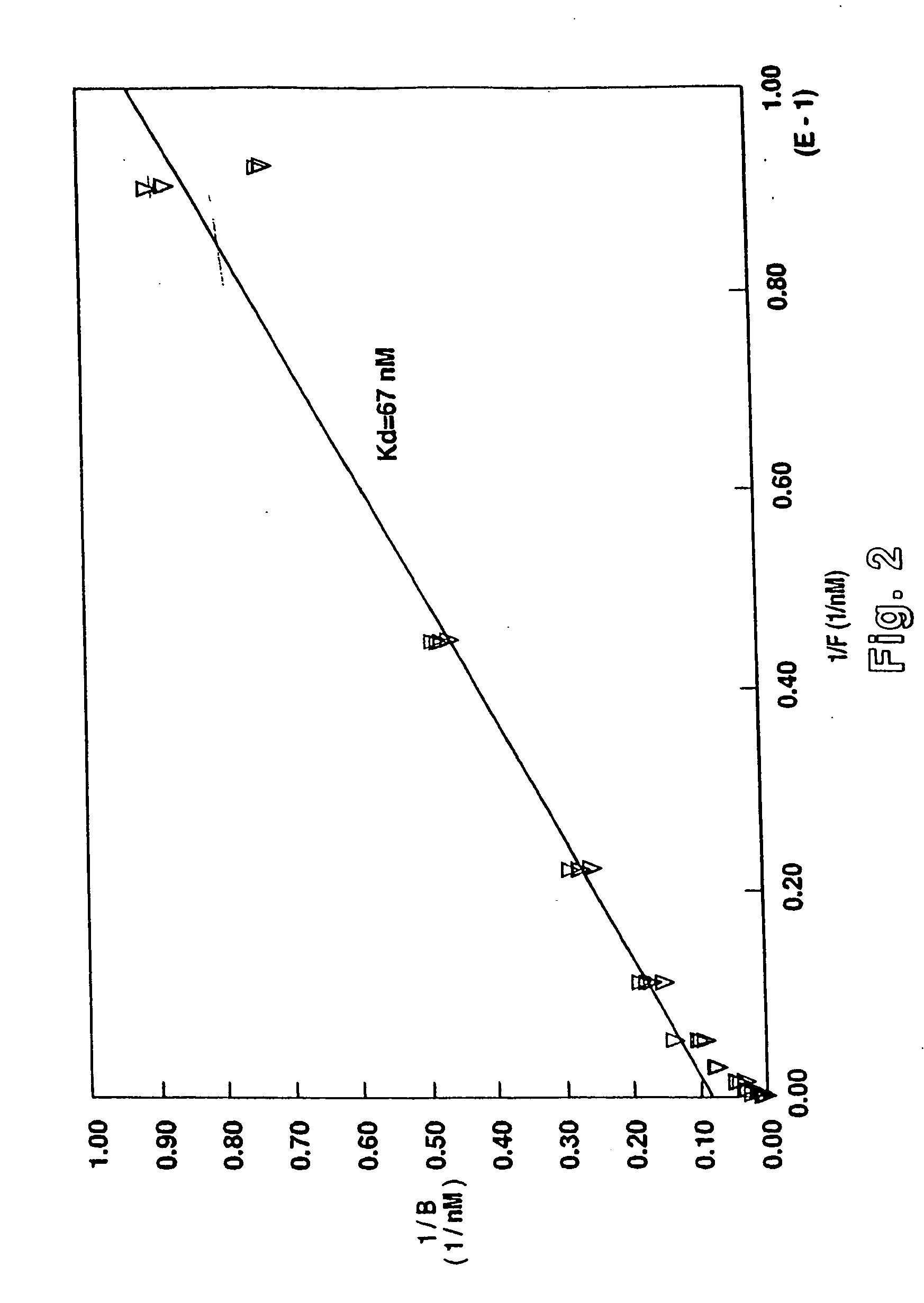
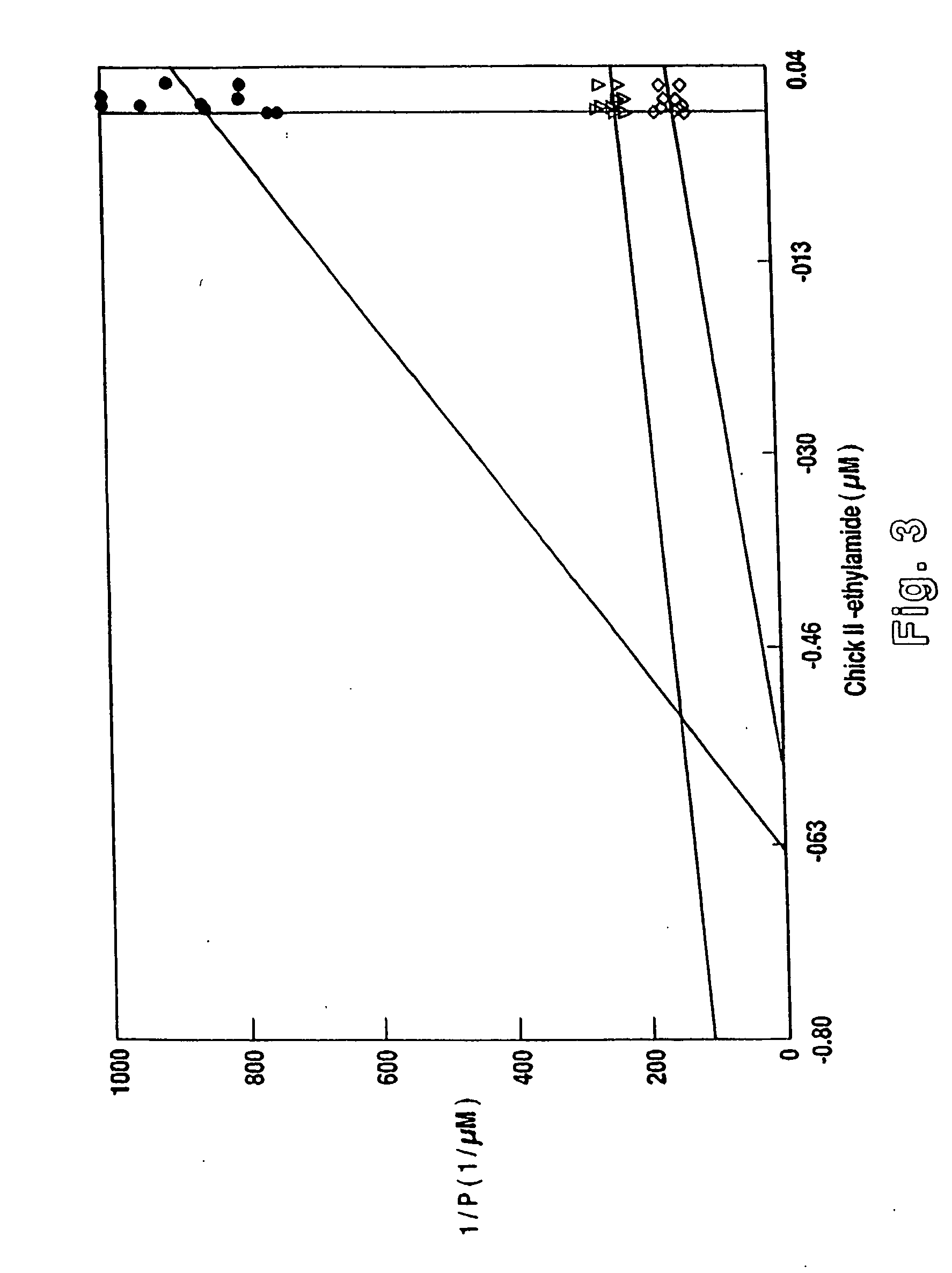
Chicken II and salmon GnRH or its analog decapeptides resistant to degradation by peptidase incorporating D-arginine, D-leucine, D-tBu-Serine, D-Trp or other active D amino acids at position 6 and ethylamide, aza-Gly-amide or other Gly amide at position 10. The non-mammalian GnRH or its analogs demonstrate preferential binding to male and female reproductive system GnRH receptors as well as tumor cell GnRH receptors in these systems. Biopotency is greater within the reproductive system and at tumor cells than at the pituitary. These non-mammalian GnRH or its analogs may be used in pharmaceutical preparations, and specifically in various treatment methods as a contraceptive or post-coital contraceptive agent. The non-mammalian GnRH or its analogs are also provided in pharmaceutical preparations that may be used clinically for maintaining pregnancy when used in very low doses and administered in pulsatile fashion, as well as in preparations for the treatment of male and female reproductive system disorders including cancers of these systems or other system with GnRH II receptors. The aza-Gly (10) amide non-mammalian analogs are yet other embodiments of the non-mammalian GnRH analogs provided as a part of the invention.
Owner:SILER KHODR THERESA
Gel prepared from biomass pyrolysis fluid and application thereof to preparation of gynecological external use medicine
InactiveCN108785680AMaintain micro-ecological environmentMaintain the ecological environmentAntibacterial agentsAntimycoticsDiseaseGynecology
The invention discloses gel prepared from biomass pyrolysis fluid. The gel is prepared from the following ingredients by using the total weight of the gel as 100 weight parts: 5 to 60 parts of biomasspyrolysis fluid, 5 to 40 parts of traditional Chinese medicine extracts, 0.1 to 20 parts of menthol, 0.1 to 10 parts of borneol, 2 to 30 parts of gel substrates and the balance water. The pH value is3.0 to 6.0. The gel provides the foundation and examples for the preparation of female external contraceptives, or external use medicine for preventing and treating female venereal diseases, or external use medicine for preventing and treating elytritis, cervicitis and cervical erosion, and external use medicine for preventing and treating carcinoma of uterine cervix. Good clinic application prospects are realized.
Owner:杨先锋
Contraceptive
Owner:JS SAKERHETABSYST
Pessulum preparation containing drospirenone or drospirenone and estrogen
InactiveCN103372015AOrganic active ingredientsPharmaceutical delivery mechanismDrospirenoneControl release
The invention relates to a contraceptive which is a pessulum preparation containing drospirenone or drospirenone and estrogen. The pessulum comprises a controlled release membrane and one or more framework sections; and at least one framework section contains the drospirenone which can slowly release acyeterion at a constant speed within 21 days, so that the zero-order release characteristic is realized. The controlled release medicinal preparation containing the drospirenone or drospirenone and estrogen can overcome the inconvenience caused by an oral contraceptive pill and improve the compliance in taking the medicine.
Owner:NAT RES INST FOR FAMILY PLANNING
Composite absorbable/biodegradable rings for controlled drug delivery
A fiber-reinforced composite ring for the controlled release of at least one bioactive agent includes a biocompatible matrix reinforced with absorbable / biodegradable fibers capable of providing the mechanical properties needed for inserting and maintaining the ring in a body cavity for a desired period of time. Such ring system as can be used for the intravaginal, intraperitoneal, and subcutaneous delivery of at least one bioactive agent, including those used as contraceptives, antimicrobial agents, and / or antiviral agents, as well as those for the treatment of cancer.
Owner:POLY MED
Pharmaceutical preparations for contraception and for preventing the risk of congenital malformations
Owner:BAYER SCHERING PHARMA OY
Use of ccl2 to inhibit abnormal uterine bleeding
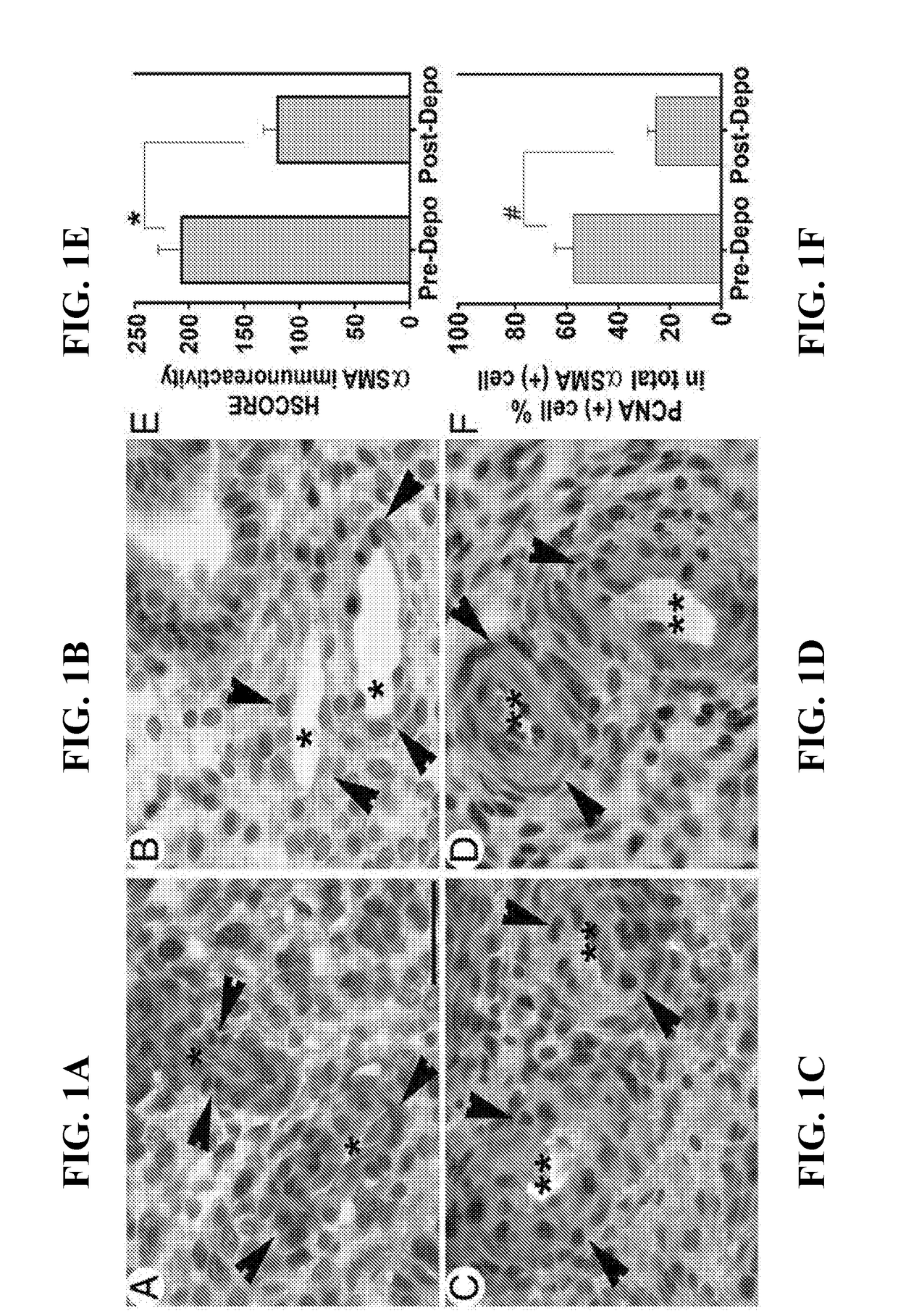
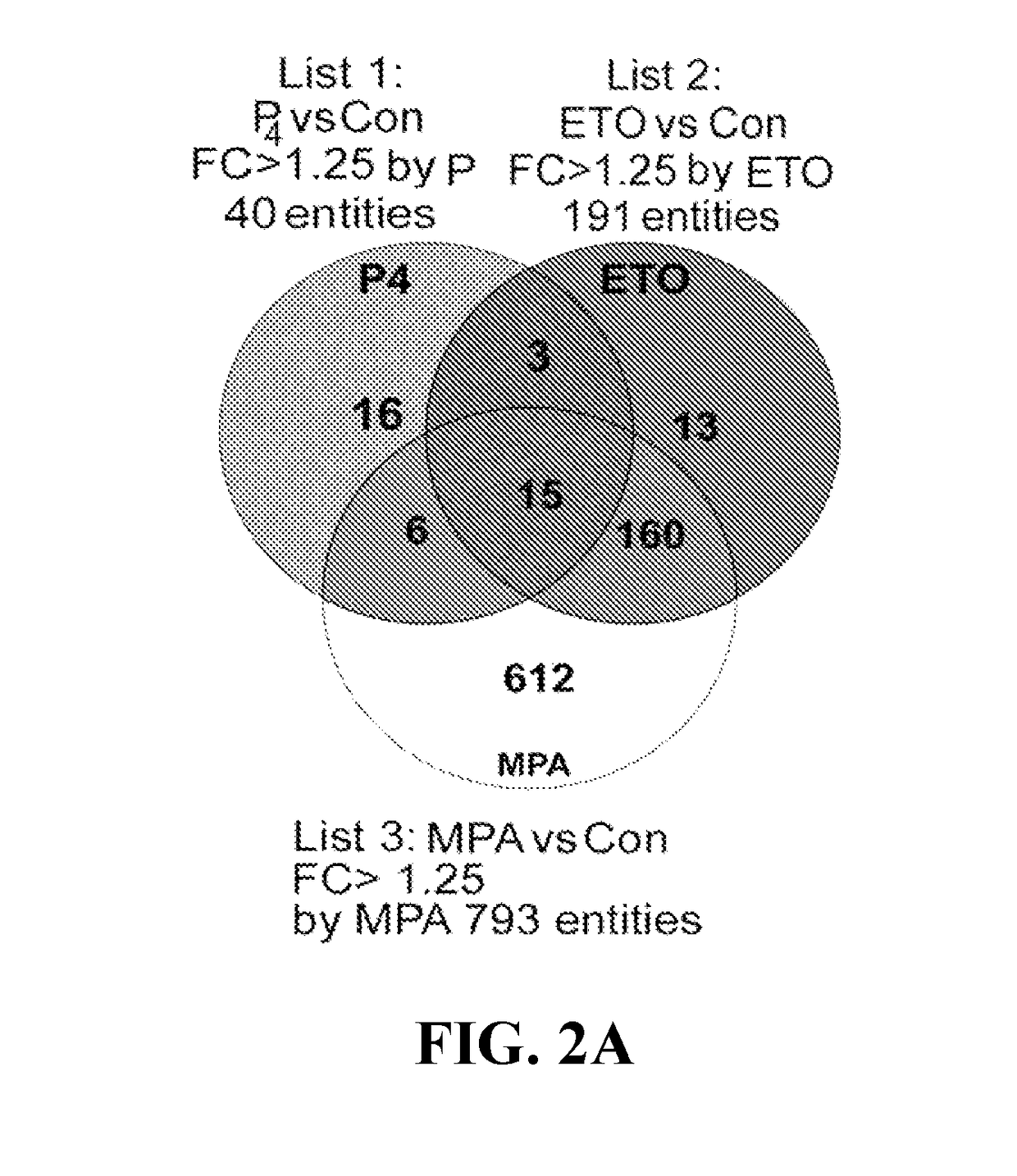
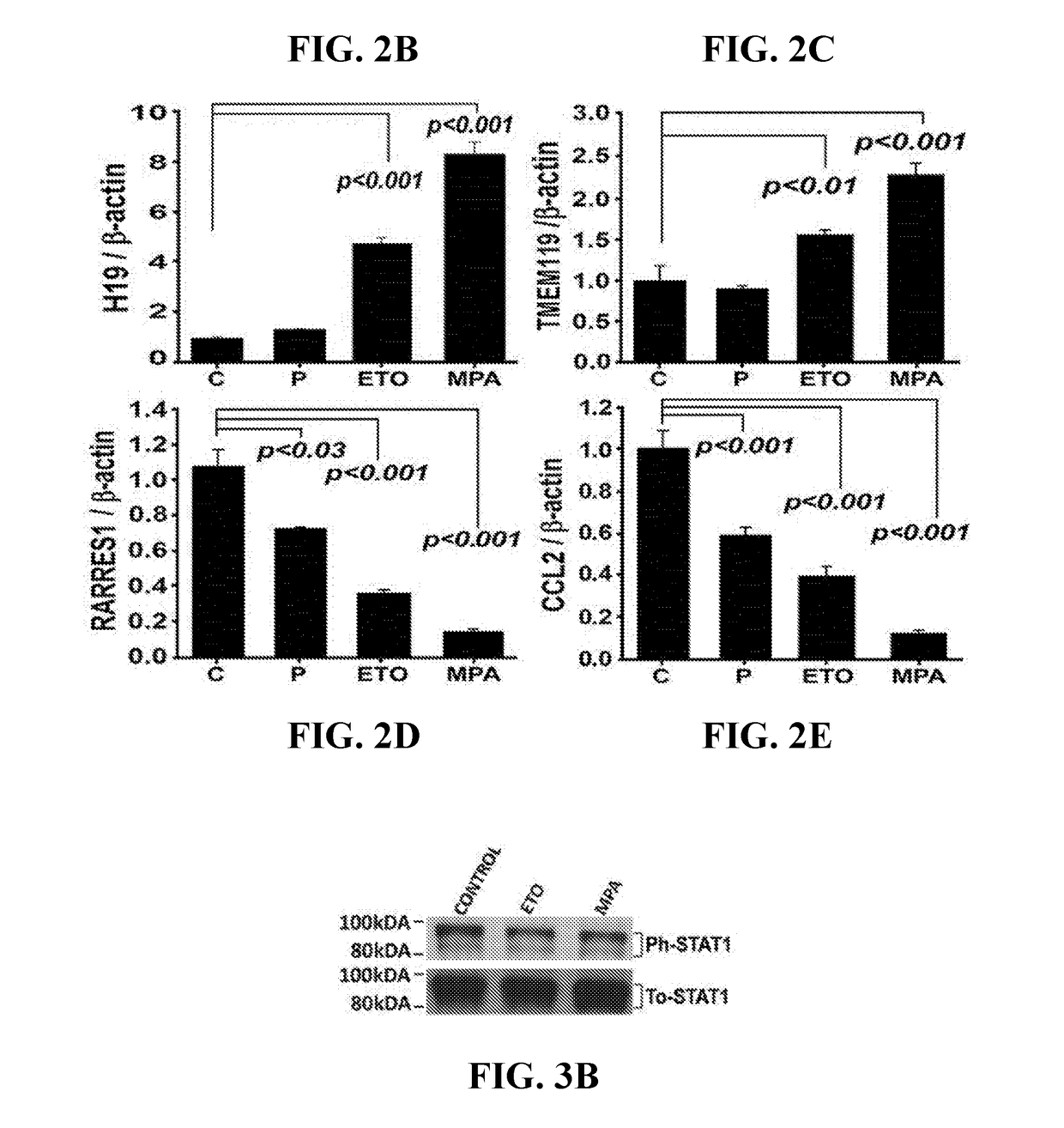
ActiveUS20180078615A1Impair vascular integrityPrevent proliferationOrganic active ingredientsPeptide/protein ingredientsCCL2Abnormal bleeding
The present invention concerns compositions and methods for inhibition of abnormal uterine bleeding (AUB), such as that associated with use of long-acting progestin-only contraceptives (LAPCs). An aspect of the invention concerns compositions comprising chemokine (C-C motif) ligand 2 (CCL2), or a biologically active fragment thereof, which may administered to subjects for inhibition of AUB. Another aspect of the invention concerns methods for inhibiting AUB, comprising administering an effective amount of CCL2, or a biologically active fragment thereof, to a subject in need thereof. Another aspect of the invention concerns a kit for inhibiting AUB.
Owner:UNIV OF SOUTH FLORIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com