Topical pharmaceutical composition comprising tenofovir, an antibacterial agent and,optonally ciclopirox
A topical drug, tenofovir technology, applied in the field of contraceptives and the preparation of the topical pharmaceutical composition, can solve the problems of no public use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
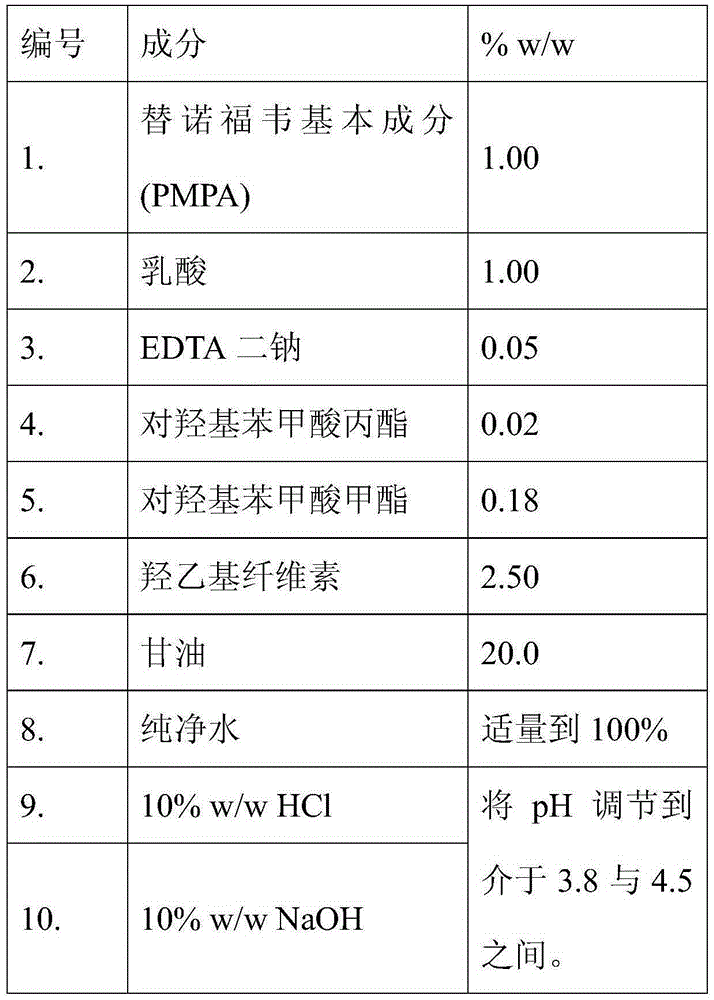
[0146]
[0147] Preparation of the organic phase:
[0148] 1. Heat the glycerin.
[0149] 2. Add and dissolve the methylparaben and propylparaben in the heated glycerin.
[0150] 3. Cool the solution obtained in step 2.
[0151] 4. Add and disperse hydroxyethyl cellulose in the solution obtained from step 3 to obtain a thick dispersion.
[0152] Preparation of the drug phase:
[0153] 1. Dissolve disodium edetate in water.
[0154] 2. Lactic acid was added and dissolved in the solution obtained in step 1.
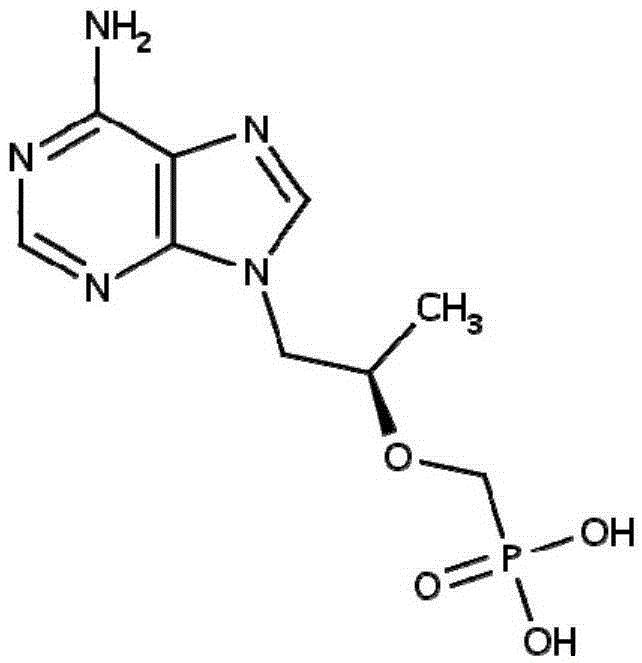
[0155] 3. Disperse tenofovir in the solution obtained in step 2.
[0156] 4. Adjust the pH to 3.8-4.5 using sodium hydroxide and hydrochloric acid until a solution of tenofovir is obtained.
[0157] Gel preparation:
[0158] 1. Add the drug phase to the organic phase under continuous stirring to obtain a gel.
Embodiment 2
[0160]
[0161]
[0162] Preparation of the organic phase:
[0163] 1. Heat the glycerin.
[0164] 2. Add and dissolve the methylparaben and propylparaben in the heated glycerin.
[0165] 3. Cool the solution obtained in step 2.
[0166] 4. Add and disperse polycarbophil in the solution obtained from step 3 to obtain a thick dispersion.
[0167] Preparation of the drug phase:
[0168] 1. Dissolve disodium edetate in water.
[0169] 2. Lactic acid was added and dissolved in the solution obtained in step 1.
[0170] 3. Disperse tenofovir in the solution obtained in step 2.
[0171] 4. Adjust the pH to 3.8-4.5 using sodium hydroxide and hydrochloric acid until a solution of tenofovir is obtained.
[0172] Gel preparation:
[0173] 1. Add the drug phase to the organic phase under continuous stirring to obtain a gel.
Embodiment 3
[0175]
[0176] Preparation of tenofovir drug phase
[0177] 1) dissolving disodium edetate in purified water;
[0178] 2) adding citric acid, lactic acid and tenofovir to the solution obtained in step (1);
[0179] 3) adding hydrochloric acid to the solution obtained in step (2) to dissolve tenofovir;
[0180] 4) adjusting the pH of the solution obtained in step (3) with sodium hydroxide solution;
[0181] 5) Add xanthan gum to the solution obtained in step (4) to form a lump-free gel.
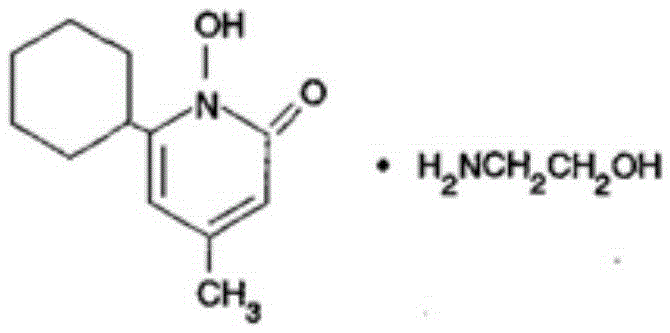
[0182] Preparation of ciclopirox olamine solution
[0183] 1) Add glycerin and propylene glycol to purified water and heat;
[0184] 2) Add methylparaben, propylparaben, cocoyldiethanolamine and polysorbate 60 to the solution obtained in step (1).
[0185] Gel preparation:
[0186] 1) The drug phase is added to the organic phase under continuous stirring to obtain a gel and the required volume is made up with purified water and the pH is measured.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com