Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
a technology of active drugs and pharmaceutical compositions, applied in the field of contraceptive kits, pharmaceutical compositions and methods, can solve the problems of poor general tolerance and low contraceptive reliability, and achieve the effect of inhibiting ovulation and inhibiting ovulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Tablets
[0325]a. Preparation of Drospirenone
[0326]Drospirenone was prepared according to a process similar to that described in WO2006 / 061309. In order to obtain DRSP with an appropriate particle size distribution, DRSP was subjected to an additional process of precipitation as mentioned in the present application.
[0327]Five batches of DRSP were prepared by variants of the above-mentioned precipitation process.
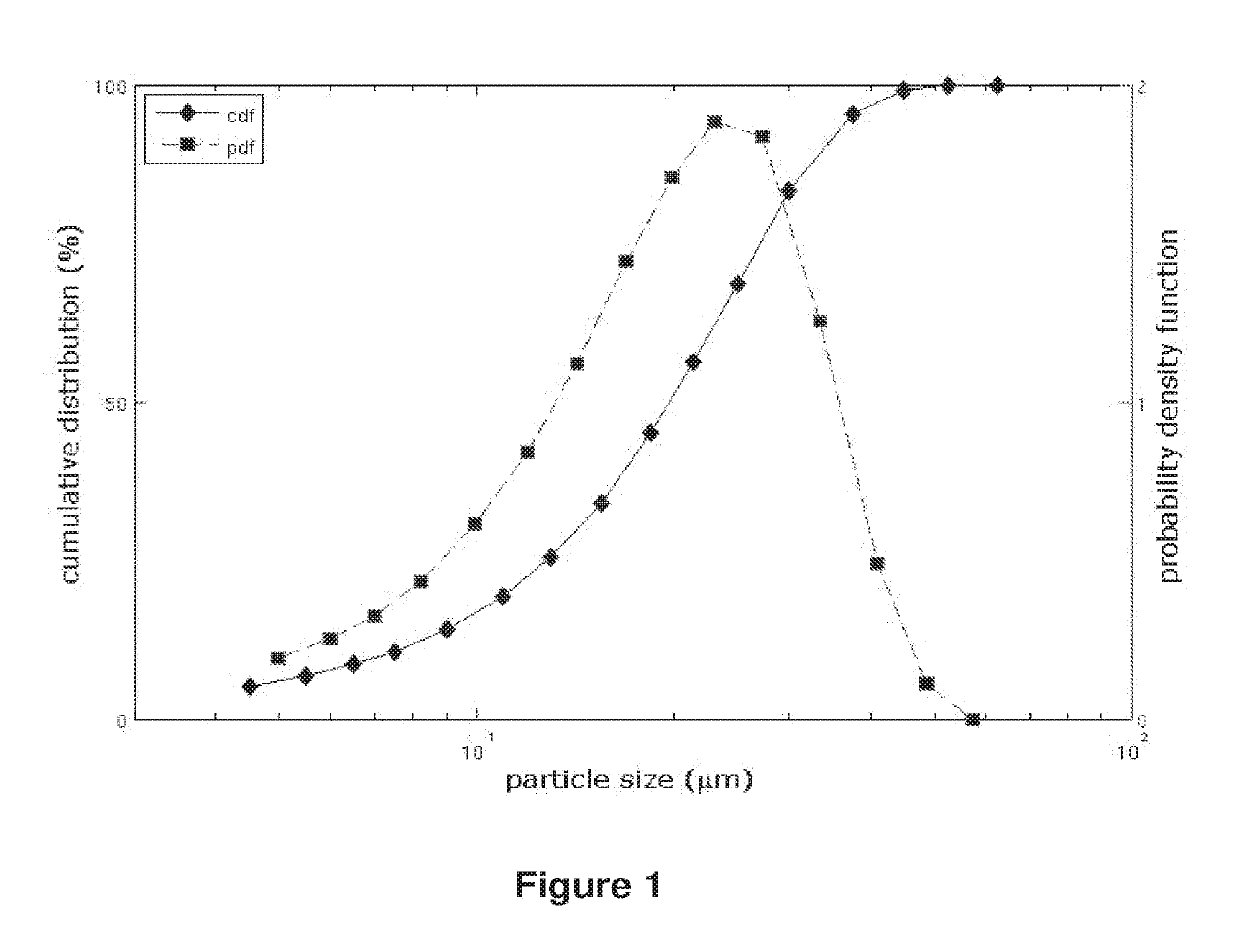
[0328]The analysis of the particle size distribution of each batch was performed by laser diffraction method in wet dispersion (Helos sensor, Sympatec with the wet disperser Quixel). The dispersant used was water. The full particle dispersion was ensured by ultrasonication.
[0329]The specific area was determined by the BET method. The results obtained are shown in table 1 below.
TABLE 1particle size distribution parameters and specific areaof DRSP batchesDRSP BatchPR100003080169080204080257080053d50 (μm)22.424.513.112.619.8d90 (μm)37.437.124.823.434.2d10 (μm)5.92.94.45.37.2...
example 2
Dissolution Profiles
a. Comparison of Tablets A-3 mg (DRSP) with Yasminelle® (Comparative)
[0333]The rate of dissolution of drospirenone from the tablets prepared in Example 1 (A-3mg) was determined by the USP XXIII Paddle Method using a USP Dissolution Test Apparatus 2 including 6 covered glass vessels and 6 paddles.
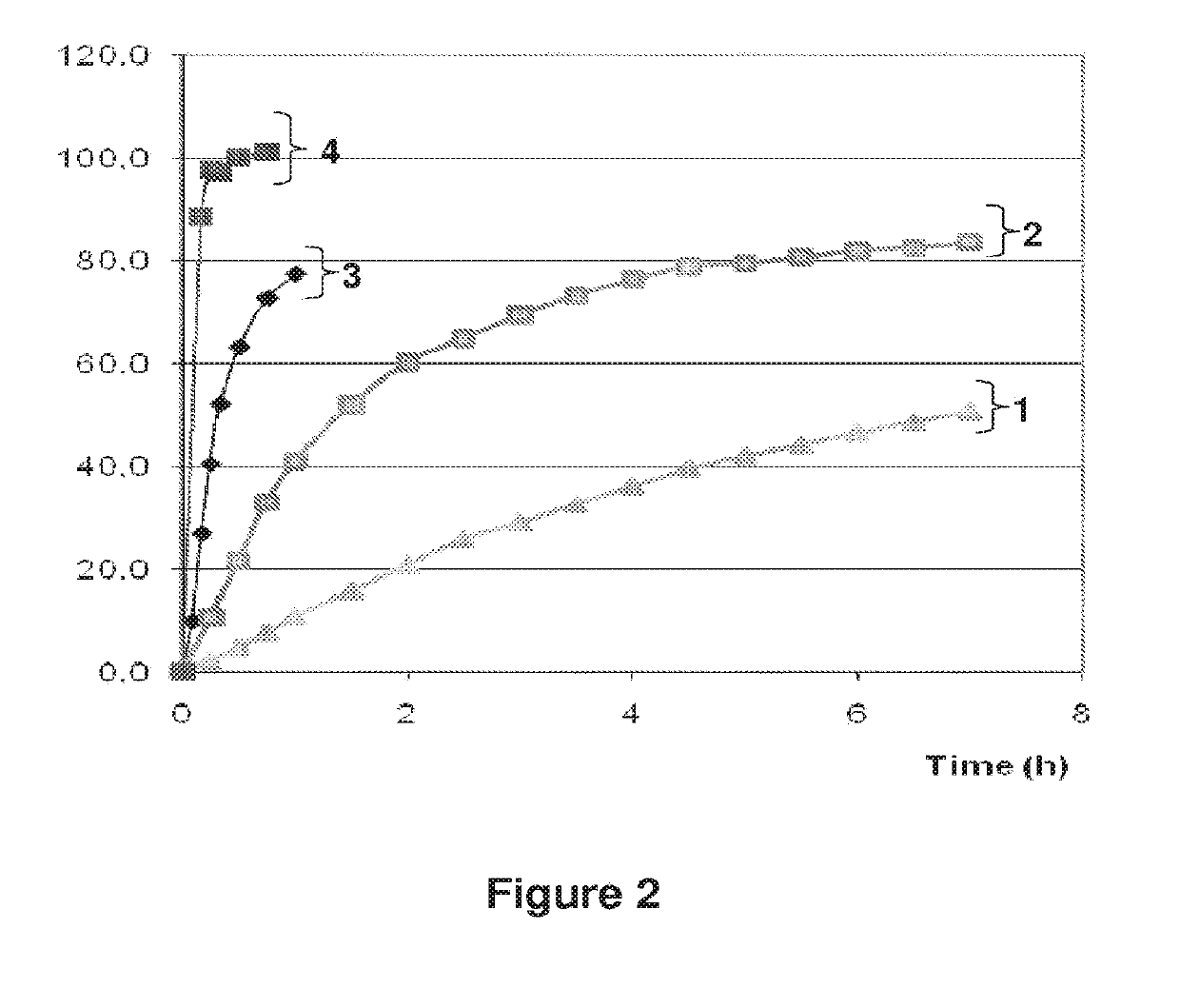
[0334]Tablets were placed in 900 ml water at a temperature of 37° C.±(0.5° C.) and stirred at 50 rpm. The amount of drospirenone released in water was measured over several hours. The mean percentages of DRSP released (which were related to the amount of drospirenone initially present in the each tablet) were calculated and plotted versus time in order to provide the in vitro dissolution profile of DRSP.
[0335]The in vitro dissolution profile of tablets A-3mg (inventive) is shown in FIG. 2 (see curve n° 2).
[0336]FIG. 2 also provides the dissolution profile obtained for Yasminelle®—tablets which comprised micronized DRSP (comparative) (see curve n° 4).
[0337]Surprisingly, th...
example 3
inetic Studies
Part 1: Evaluation of the Pharmacokinetics Parameters for the Composition According to the Invention (Tablet A-3 mg) as Compared to Yasminelle®
Objectives
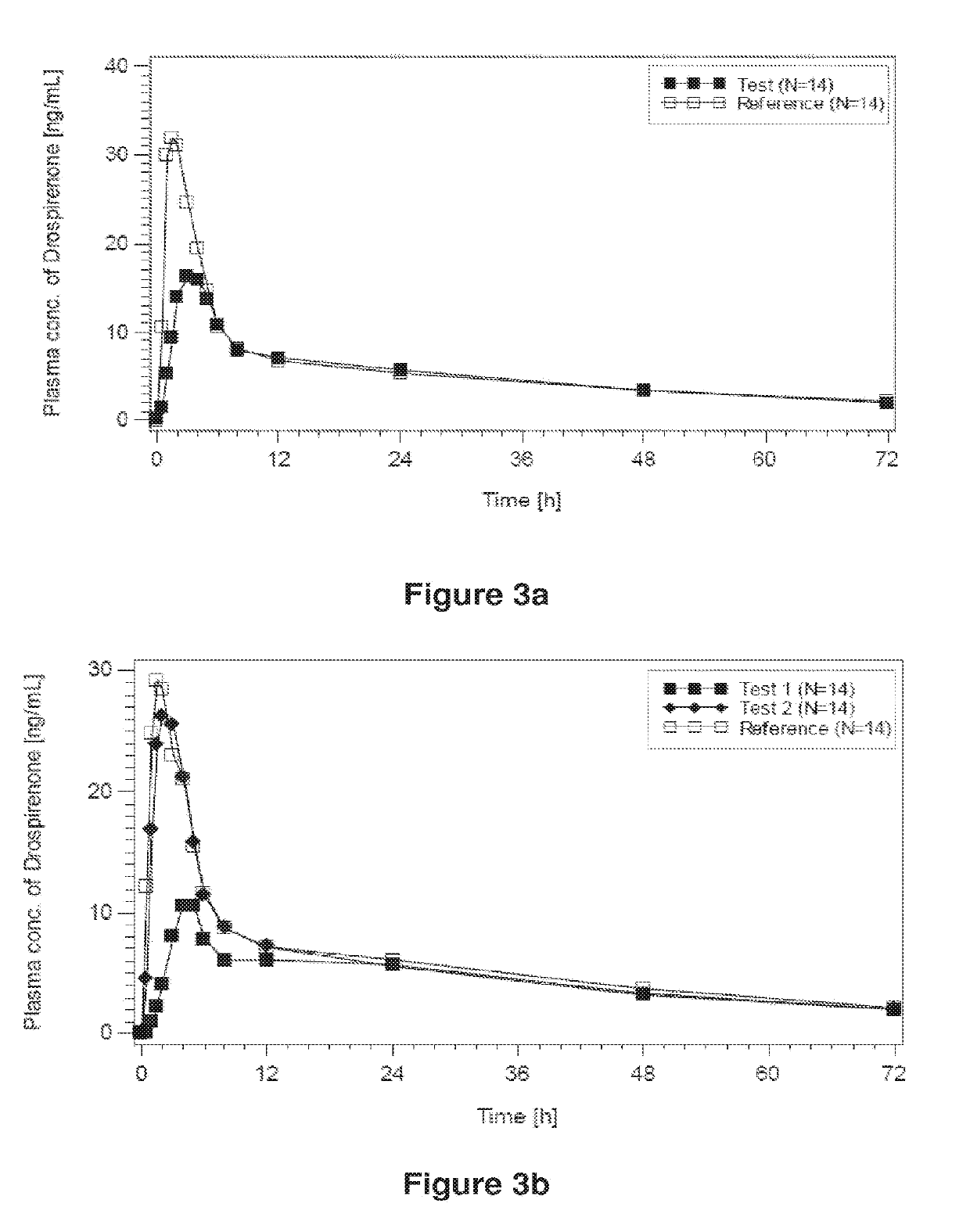
[0341]The main objective of the present trial was to assess the bioavailability of an oral test preparation containing drospirenone at 3.0 mg (tablets described in Example 1 obtained from batch 080053 (i.e. A-3mg), called hereunder “test product” hereunder) as compared to a market standard (Yasminelle®, Schering AG, called hereunder “reference product”) after oral administration of a single dose of drospirenone at 3.0 mg under fasting conditions in two different periods, 7 days apart. Yasminelle® comprises 3.0 mg DRSP in micronized form and 0.030 mg of ethinylestradiol.
[0342]In order to investigate the relative bioavailability of the products, the 90% confidence intervals for the intra-individual ratios (test vs. reference) for the endpoint(s) (AUC0-tlast and Cmax of drospirenone) were determined.
[0343]The secondary ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| d50 particle size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com