Capsaicin beta-cyclodectrin inclusion-compound and liposome and gel of inclusion-compound
A technology of cyclodextrin inclusion compound and capsaicin, which is applied in the direction of liposome delivery, non-active ingredients of polymer compounds, drug combination, etc., can solve problems such as flushing, low solubility in water, strong irritation of cream, etc., and achieve The quality is stable and easy to control, the production process is simple, and the effect of improving the skin penetration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12
[0030] Preparation and Apparent Solubility Determination of Capsaicin β-Cyclodextrin Inclusion Compound
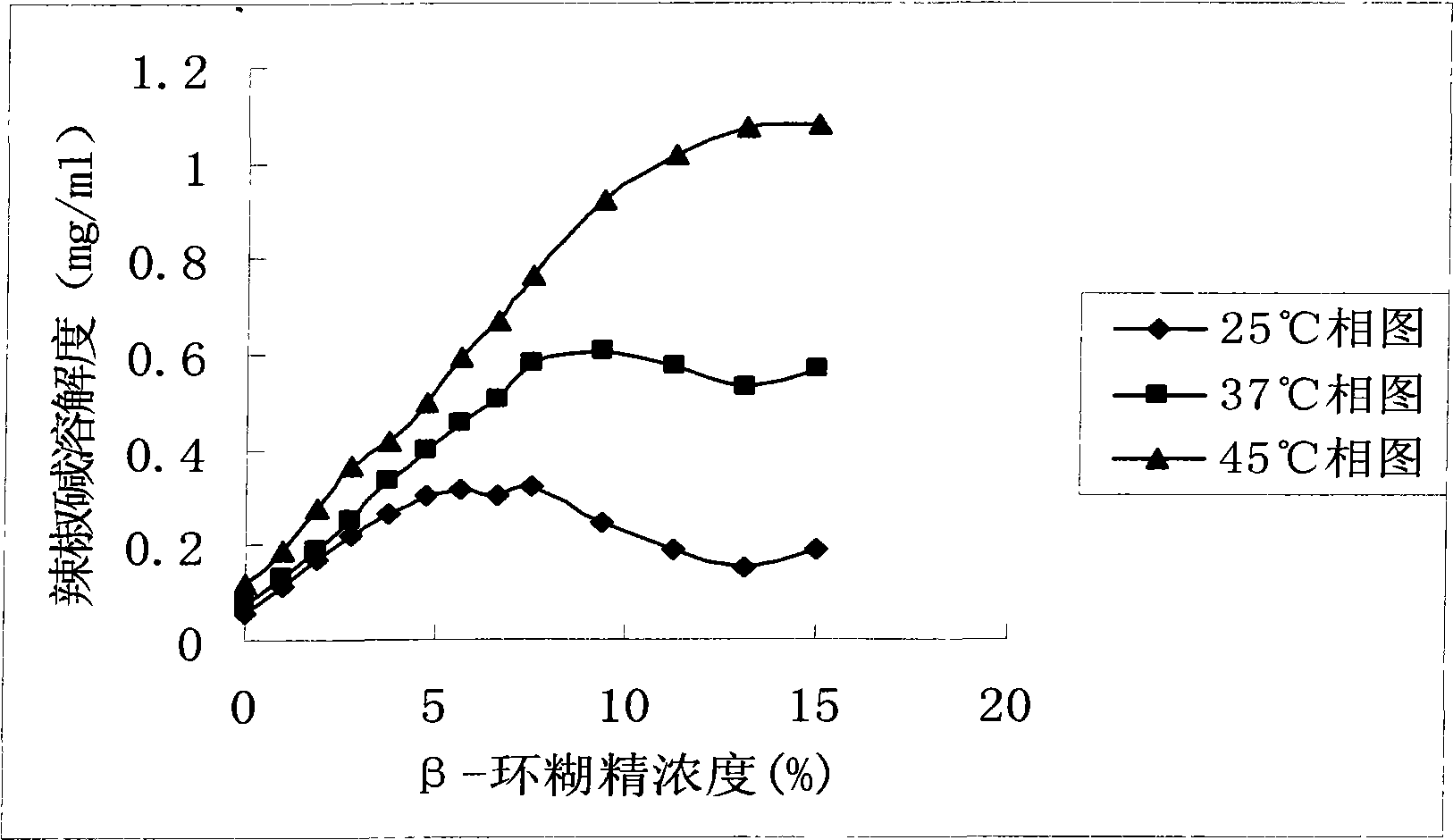
[0031] Weigh an appropriate amount of β-cyclodextrin according to Table 1, pour it into 100ml of distilled water, stir to dissolve; take another 0.075g of capsaicin, and pour it into the above-mentioned β-cyclodextrin solution; Stir for 4 hours to obtain a solution capsaicin β-cyclodextrin inclusion compound; freeze-dry to obtain a solid capsaicin β-cyclodextrin inclusion compound. Place the prepared capsaicin β-cyclodextrin inclusion compound under the temperature conditions of 25°C, 37°C, and 45°C respectively, add appropriate amount of water, take a sample after equilibrating for 72 hours, filter it with a 0.45 μm microporous membrane, and directly Inject into high-performance liquid chromatograph and analyze, calculate the apparent solubility of capsaicin, the result is as follows figure 1 shown.
[0032] Table 1 The amount of β-cyclodextrin used in Examples 1-12 (%)...
Embodiment 13~15
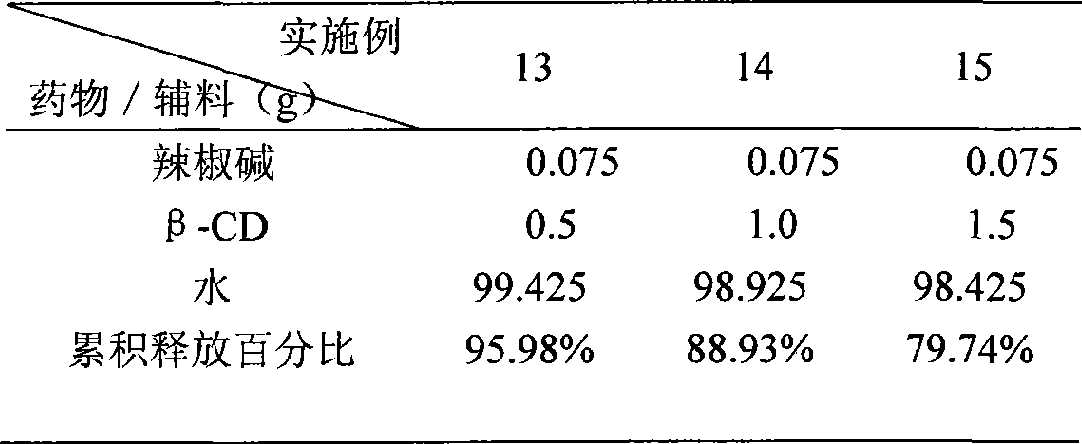
[0035] Preparation and in vitro release evaluation of capsaicin β-cyclodextrin inclusion complex
[0036] Weigh an appropriate amount of capsaicin and β-cyclodextrin (β-CD) into water, shake in a water bath shaker at a constant temperature (100 cycles / min) at 37°C for 24 hours to obtain capsaicin β-cyclodextrin inclusion compound. Take the dialysis membrane and install it on the Franz diffusion cell, add 7.0mL ethanol-7.4PBS solution into the receiving cell as the receiving solution, keep the temperature at (37±1)°C, add 2mL of capsaicin inclusion compound sample into the supply cell. Samples were taken at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 hours, and the capsaicin content of the samples was determined by HPLC to calculate the cumulative drug penetration. At the same time, 0.075% capsaicin cream and hydrogel were used as controls. The results showed that the 4-hour cumulative release percentage of the inclusion compound was greater than that of capsaicin cream (30%) and hydro...
Embodiment 16~ Embodiment 27
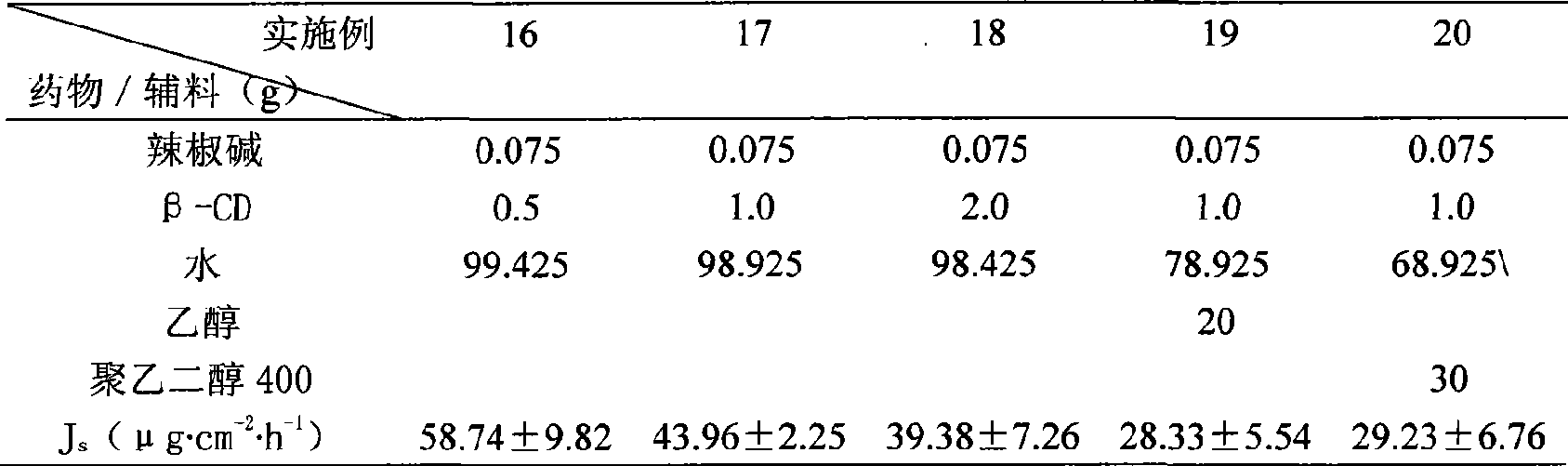
[0040]Preparation of capsaicin β-cyclodextrin inclusion complex and its gel and evaluation of percutaneous permeability in vitro
[0041] Add capsaicin, β-cyclodextrin and other auxiliary materials weighed according to the amount into water, and shake in a water bath shaker at a constant temperature (100 cycles / min) at 37°C for 24 hours to obtain capsaicin β-cyclodextrin inclusion compound. Another prescribed amount of carbomer is added to the clathrate solution, stirred evenly, then triethanolamine is added, stirred, and left to stand until the carbomer swells completely to obtain capsaicin β-cyclodextrin inclusion compound gel. The isolated mouse skin was installed on the Franz diffusion cell, the receiving solution and the temperature were maintained as above, and 2 mL of capsaicin inclusion compound and capsaicin inclusion compound gel were respectively added to the supply cell. Samples were taken at 2, 4, 6, 7, 8, 9, 10 and 11 hours respectively, and the capsaicin content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com