Transdermal paster containing bulleyaconitine A and its preparation method
A technology of aconitin and transdermal patch, which is applied in the field of transdermal patch of aconitin and its preparation, can solve the problems of high skin irritation and poor stability, and achieve safety and ease of use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The transdermal patch described in this embodiment is a drug storage (adhesive skeleton type) patch, which consists of a backing layer, a protective layer and a drug-containing adhesive skeleton three-layer structure, and the materials used in each layer are as follows:
[0023] Backing layer: Polyurethane film
[0024] Protective layer: PTFE film
[0025] Drug storage (drug-containing adhesive matrix):
[0026] Drug storage prescription (100 stickers):
[0027] Silicone PSA 16g
[0028] Aconitin 0.4g
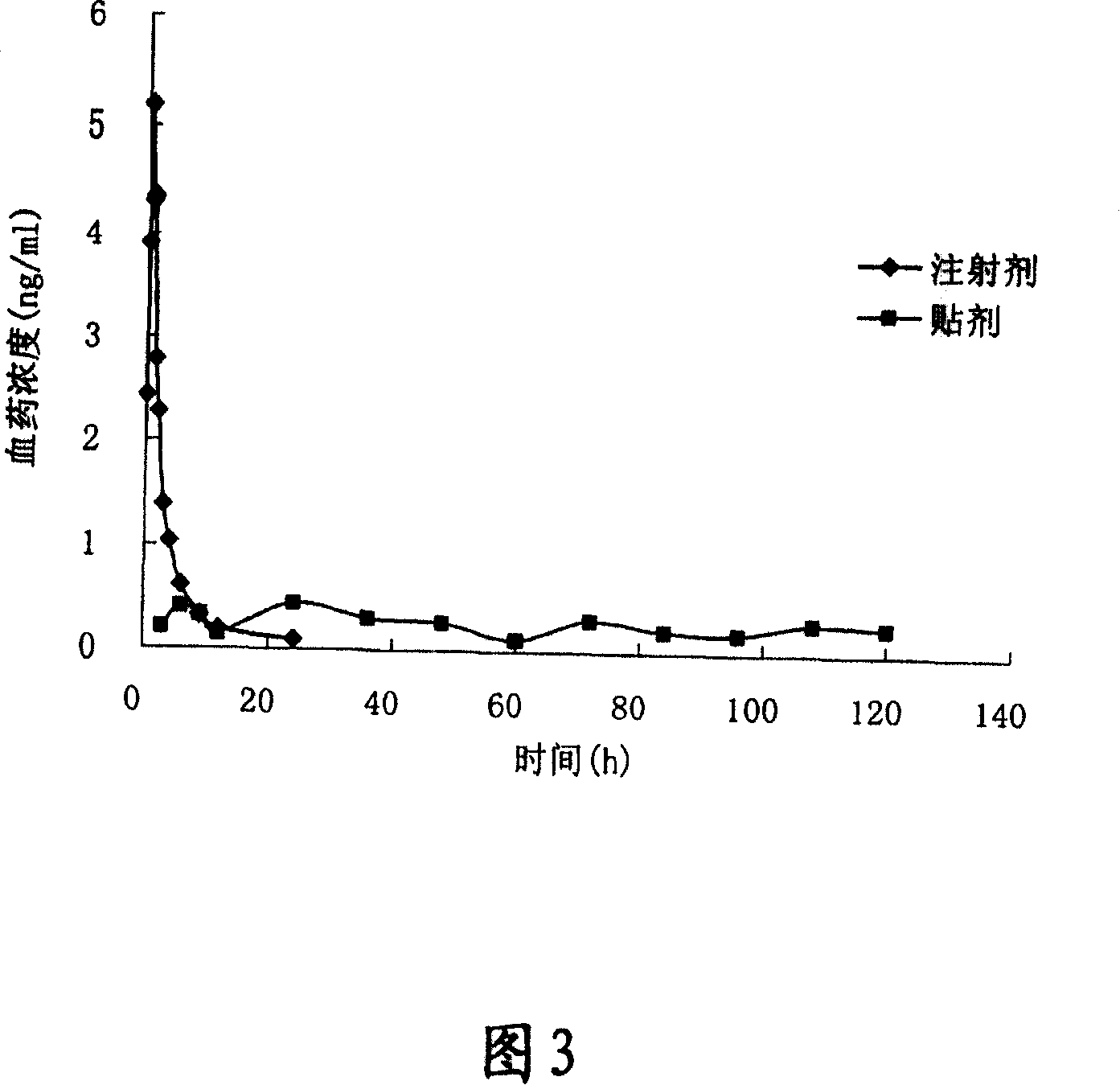
[0029] Preparation process: Take the prescribed amount of aconitin and silicone pressure-sensitive adhesive, add appropriate amount of ethyl acetate to dissolve, adjust to a suitable consistency, apply on the protective layer, control the thickness of the coating, dry, and cover the back Lining, cut to the appropriate shape and size. Control patch area is 10cm 2 , Each patch contains 4mg of medicine.
[0030] The time lag of the aconitin patch described in this em...
Embodiment 2
[0032] The transdermal patch described in this example is an adhesive skeleton patch, consisting of a three-layer structure of a backing layer, a protective layer, and a drug-containing adhesive skeleton. The materials used in each layer are as follows:
[0033] Backing layer: non-woven fabric
[0034] Protective layer: PTFE film
[0035] Drug-containing adhesive matrix:
[0036] Adhesive frame prescription (100 stickers):
[0037] Silicone pressure sensitive adhesive 10g
[0038] Aconitin 0.6g
[0039] Preparation process: Take the prescribed amount of aconitin and silicone pressure-sensitive adhesive, add an appropriate amount of ethyl acetate to dissolve, adjust to a suitable consistency, coat on the protective layer, control the thickness of the coating, dry, and cover the back Lining, cut to proper shape. Control patch area is 10cm 2 , Each patch contains 6mg of medicine.
[0040] The time lag of the aconitin patch described in this embodiment is 10.5h, and the tra...
Embodiment 3
[0042] The transdermal patch described in this example is an adhesive skeleton patch, consisting of a three-layer structure of a backing layer, a protective layer, and a drug-containing adhesive skeleton. The materials used in each layer are as follows:
[0043] Backing layer: non-woven fabric
[0044] Protective layer: anti-stick paper
[0045] Drug-containing adhesive matrix:
[0046] Adhesive frame prescription (100 stickers):
[0047] Polyacrylate pressure sensitive adhesive 20g
[0048] Aconitin 1.5g
[0049] Preparation process: Take the prescribed amount of aconitin and polyacrylate pressure-sensitive adhesive, add appropriate amount of ethyl acetate to dissolve, adjust to a suitable consistency, coat on the protective layer, control the thickness of the coating, dry, and cover Backing layer, cut to appropriate shape. Control patch area is 10cm 2 , Each patch contains 15mg of medicine.
[0050] The time lag of the aconitin patch described in this embodiment is 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com