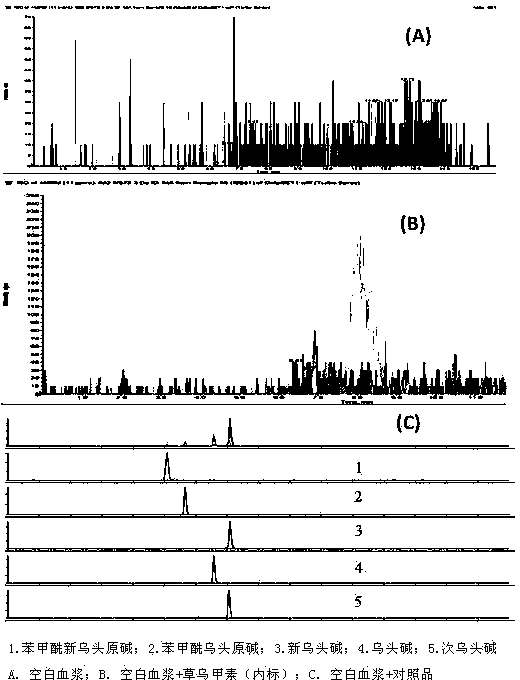
Method for simultaneously determining 5 alkaloids in Sini agent vegetable drug plasma
A botanical and plasma technology, applied in measurement devices, instruments, scientific instruments, etc., can solve difficult quality control, formulation design, clinical rational drug use, no simultaneous determination method, lack of pharmacokinetics of Sini Decoction botanicals, etc. problems, to achieve the effect of sensitive analytical methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0015] Experimental example 1 Non-internal standard method linear relationship investigation
[0016] Take 7 parts of blank plasma, each 100 μl, add different concentrations of standard solution, so that the concentration of aconitine in the plasma is 0.06, 0.6, 3, 6, 30, 60, 300 ng·mL -1 , the concentrations of neoaconitine were 0.102, 1.02, 5.1, 10.2, 51, 102, 510 ng·mL -1 , Hypoaconitine 0.13, 1.3, 6.5, 13, 65, 130, 650 ng·mL -1 , the concentration of benzoyl neoaconitine is 0.1, 1, 5, 10, 50, 100, 500 ng·mL -1 , the concentrations of benzoylaconitine were 0.12, 1.2, 6, 12, 60, 120, 600 ng·mL -1 , to prepare a series of standard solutions.
[0017] Take the concentration of each reference substance as the abscissa and the peak area of the reference substance as the ordinate, and use weighted least square regression (weight coefficient 1 / C) to obtain the regression equation of each reference substance. The regression curve results show that:
[0018] Aconitine at ...
experiment example 2
[0019] Experimental example 2 Investigation of linear relationship with internal standard method
[0020] Take 7 parts of blank plasma, each 100 μl, add different concentrations of standard solution, so that the concentration of aconitine in the plasma is 0.06, 0.6, 3, 6, 30, 60, 300 ng·mL -1 , the concentrations of neoaconitine were 0.102, 1.02, 5.1, 10.2, 51, 102, 510 ng·mL -1 , Hypoaconitine 0.13, 1.3, 6.5, 13, 65, 130, 650 ng·mL -1 , the concentration of benzoyl neoaconitine is 0.1, 1, 5, 10, 50, 100, 500 ng·mL -1 , the concentrations of benzoylaconitine were 0.12, 1.2, 6, 12, 60, 120, 600 ng·mL -1 , to prepare a series of standard solutions.
[0021] Take the ratio of the concentration of each reference substance to the concentration of the internal standard X as the abscissa, and take the peak area of the reference substance and the percentage of the peak area of the internal standard Y as the ordinate, and use Y to X for weighted least squares regression (wei...
experiment example 3
[0023] Experimental example 3 Precision and recovery investigation
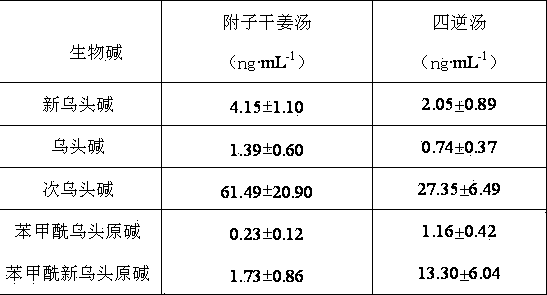
[0024] Take 100 μl of blank plasma, add aconitine (internal standard) solution and different concentrations of reference solution, and prepare each containing aconitine, neoaconitine and hypoaconitine, benzoyl neoaconitine, Benzoyl aconitine 1, 10, 100 ng·mL -1 3 concentrations of standard drug-containing plasma, 5 samples were made for each concentration, and the methodological precision was determined.
[0025] Another blank plasma was taken to obtain the blank plasma supernatant, and then different amounts of standard solution and aconitin solution were added to obtain aconitine, neoaconitine, hypoaconitine, and benzoyl neoaconitine. Base, benzoyl neoaconitine 1, 10, 100 ng·mL -1 the control solution. Injection analysis, calculation of extraction recovery. See Table 1. It can be seen from the table that the precision and extraction recovery experimental results of aconitine, neoaconitine, hypoaconi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com