Bulleyaconitine A micro-balloons and bulleyaconitine A long-acting injection and preparation method and application of same
A technology of aconitin and aconitin, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, anti-inflammatory agents, etc., which can solve the problems of large fluctuations in blood drug concentration, application restrictions, and adverse reactions and other issues, to achieve the effect of reducing the number of administration, good safety and high patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Prescription of Microspheres
[0051] Aconitin 3g
[0052] Glycolide-Lactide Copolymer 12g
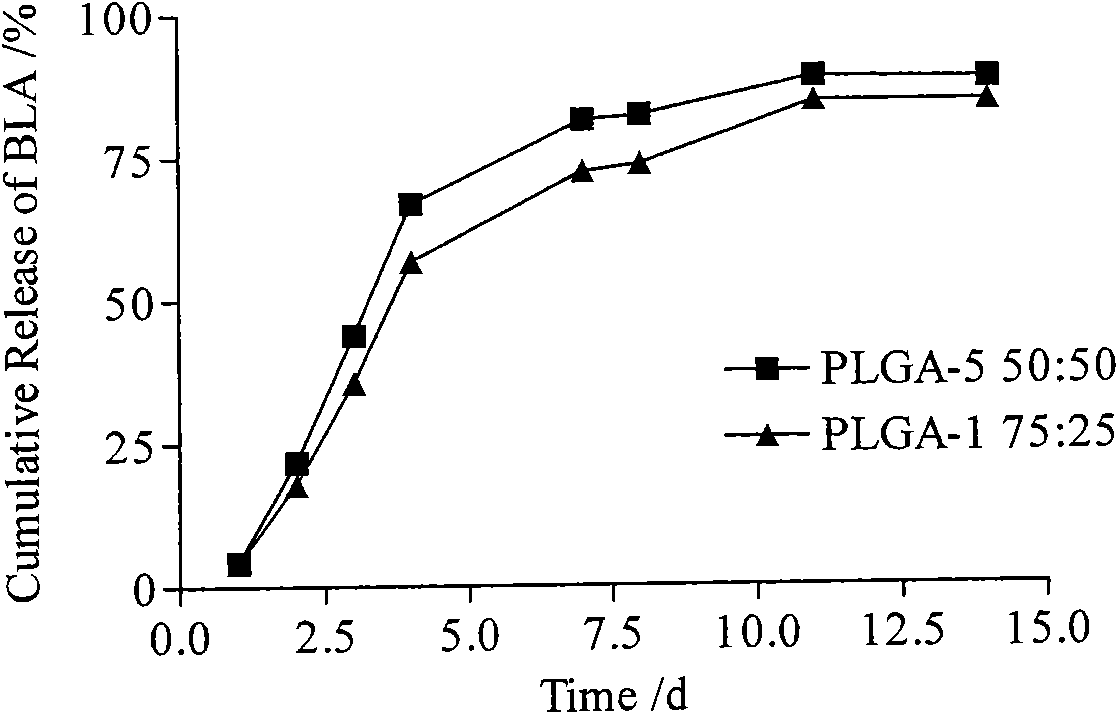
[0053] In the glycolide-lactide copolymer, the molar ratio of the monomers is 50:50, the weight average molecular weight is 5000 Daltons, and the average particle diameter of the microspheres is 200 μm;
[0054] Preparation:
[0055] The device used in the preparation method can refer to the device disclosed in the Chinese patent, Patent No. 200420081682.5, and the diameter of the nozzle hole of the nozzle is 1mm;
[0056] Dissolving aconitin and glycolide-lactide copolymer in dichloromethane to prepare a solution with a total solid weight content of 30%, filtered through a filter with a pore size of 0.45 μm and used as a dispersed phase;
[0057]Dissolving polyvinyl alcohol 04-86 in water to make an aqueous solution with a weight content of 0.1%, filtered through a filter with a pore size of 5 μm, and used as the water phase;
[0058] The disperse phase and the water phase ...
Embodiment 2
[0060] Prescription of microspheres:
[0061] Aconitine 10g
[0062] Polylactide 10g
[0063] In the glycolide-lactide copolymer, the molar ratio of the monomers is 25:75, the weight average molecular weight is 100,000 Daltons, and the average particle size of the microspheres is 1 μm;
[0064] Preparation:
[0065] The device used in the preparation method can refer to the device disclosed in the Chinese patent, Patent No. 200420081682.5, and the diameter of the nozzle hole of the nozzle is 0.1mm;
[0066] Dissolving aconitin and polylactide in ethyl acetate to prepare a solution with a total solid weight content of 10%, filtered through a filter with a pore size of 0.45 μm and used as a dispersed phase;
[0067] Dissolving polyvinyl alcohol 05-88 in water to make an aqueous solution with a weight content of 5%, filtered through a filter with a pore size of 5 μm, and used as the water phase;
[0068] The dispersed phase and the aqueous phase enter the nozzle submerged in ...
Embodiment 3
[0070] Prescription for Suspension Injection:
[0071] The aconitin microspheres 50g of embodiment 1
[0072] Suspending agent 10g
[0073] Osmotic pressure regulator 1g
[0074] Wetting agent 0.2g
[0075] Water for injection 38.8g
[0076] The suspending agent is sodium carboxymethyl cellulose, the osmotic pressure regulator is sodium chloride, and the wetting agent is polysorbate.
[0077] Stir the above-mentioned various raw materials evenly, then pack them into ampoules, seal and sterilize by autoclaving at 115°C for 30 minutes, and then it can be made into a suspension injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com