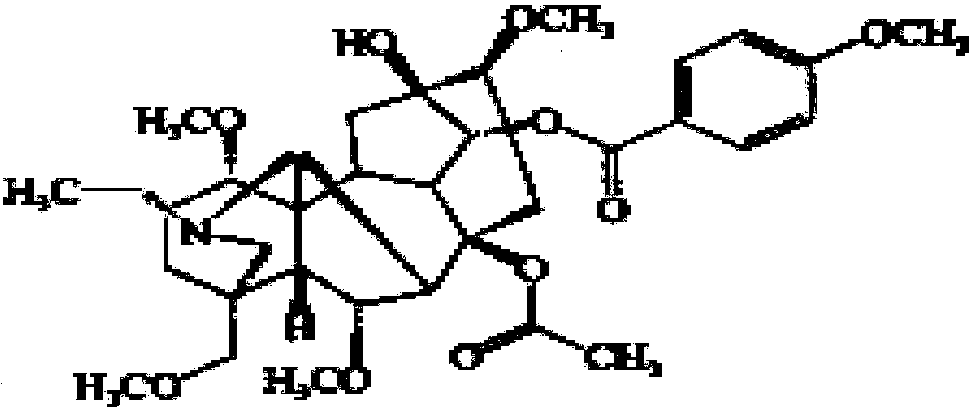
Application of bulleyaconitine A
A technology of clathrin and a drug, applied in the field of medicine, can solve the problems of easy formation of drug resistance and dependence, expensive biological preparations, etc., and achieve the effects of improving physiological indicators, reducing the area of rash and relieving itching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: mouse model test
[0017] 1. Experimental animals
[0018] SPF grade BALB / C mice, body weight: 20±2g, age: 6-8 weeks, provided by the Experimental Animal Center of Kunming Medical University, male, free to eat, temperature 25±3°C, kept in SPF grade animal room.
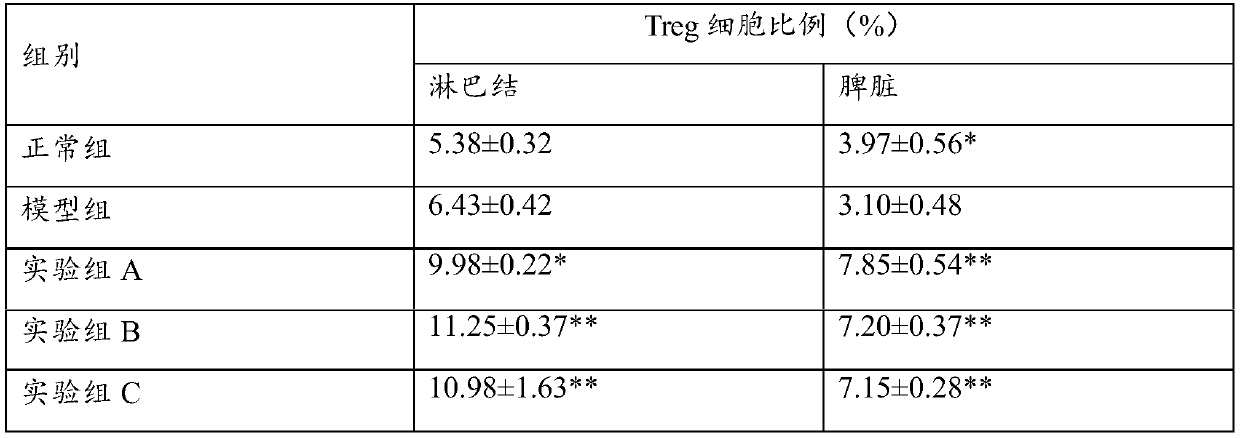
[0019] 2. Grouping of animals
[0020] 60 BALB / C mice were randomly divided into 6 groups, including normal group, model group, experimental group A, experimental group B, and experimental group C, with 12 mice in each group.
[0021] 3. Establishment of mouse psoriasis model
[0022] Use electric clippers to shave the back hair of each mouse, and then apply a depilatory cream to remove the remaining hair on the back of the mouse, exposing the skin with an area of about 3cm×2cm. Two days later, 62.5 mg of 5% imiquimod ointment was applied to the back skin of each mouse every day for 7 consecutive days. Obvious erythema, scales and thickening appeared on the back skin of the mouse, which mean...
Embodiment 2
[0062] Embodiment 2: clinical trial
[0063] 1. Research object
[0064] The observed cases came from patients who were diagnosed with psoriasis vulgaris at the Dermatology Clinic of Yunnan Provincial Hospital of Traditional Chinese Medicine from December 2017 to May 2019.
[0065] 2. Diagnostic criteria
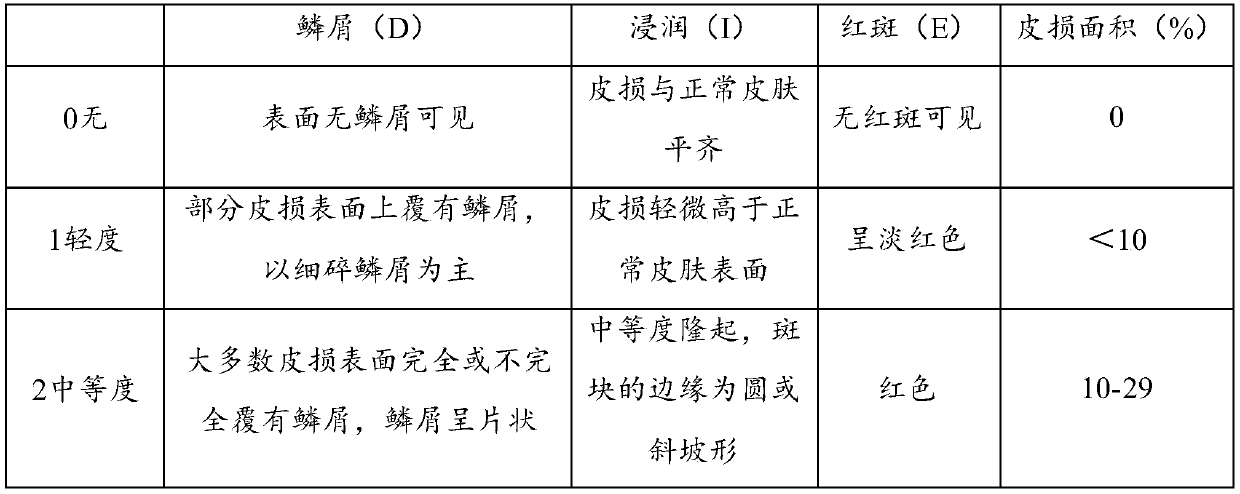
[0066] Formulated according to "Chinese Clinical Dermatology". The skin lesions are red papules and macules, which can be fused into sheets with obvious edges and covered with multiple layers of silvery white scales. After the scales are scraped off, a layer of translucent film can be seen, and punctiform bleeding can be seen after the thin scales are removed.
[0067] 2.1 Grading criteria for disease severity
[0068] The severity of psoriasis is graded according to the area of the lesion:
[0069] Mild: skin lesion area < 10% of body surface area;
[0070] Moderate disease: the area of skin lesions is 10% to 30% of the body surface area;
[0071] Severe cases: sk...
Embodiment 3
[0185] Embodiment 3: Aconitin safety test
[0186] Under the GLP experimental conditions of the Chongqing Institute of Traditional Chinese Medicine (Chongqing Drug Safety Evaluation Center), the SD rats were gavaged with Aconitin Tablets for 3 months. The multiple administration toxicity test data showed that the no toxic reaction dose of Aconitin ( NOAEL) is 0.15mg / kg, the safe dose is 0.3mg / kg, and the adult and rat dose conversion is about 6.15 times different, then the adult NOAEL (NOAEL) is 0.024mg / kg, and the adult safe dose is 0.049mg / kg; if an adult is calculated as 60kg, the NOAEL for adults is 1.44mg / 60kg, and the safe dose for adults is 2.94mg / 60kg. The specific test is as follows:
[0187] Experimental observation indicators: During the experiment, observe the general conditions of the animals, such as appearance signs, behavioral activities, food intake, drinking water, urine, and feces, etc.; weigh the body weight and food intake once a week; detect hematology ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com