Medical use of pentacyclic triterpenoid saponin and pharmaceutical composition thereof
A technology of pentacyclic triterpene saponins and compositions, which is applied in the field of medicine and can solve problems such as unclear prevention and treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
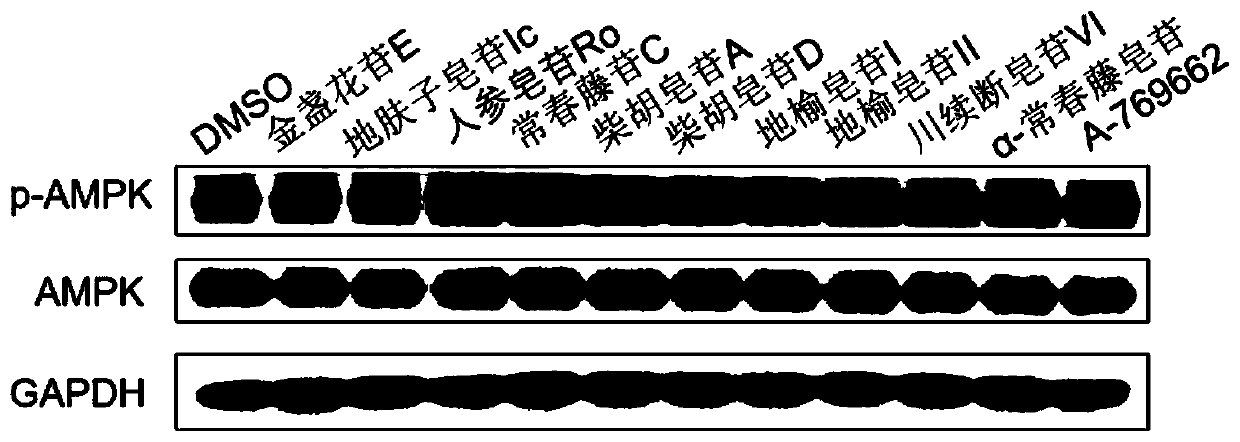
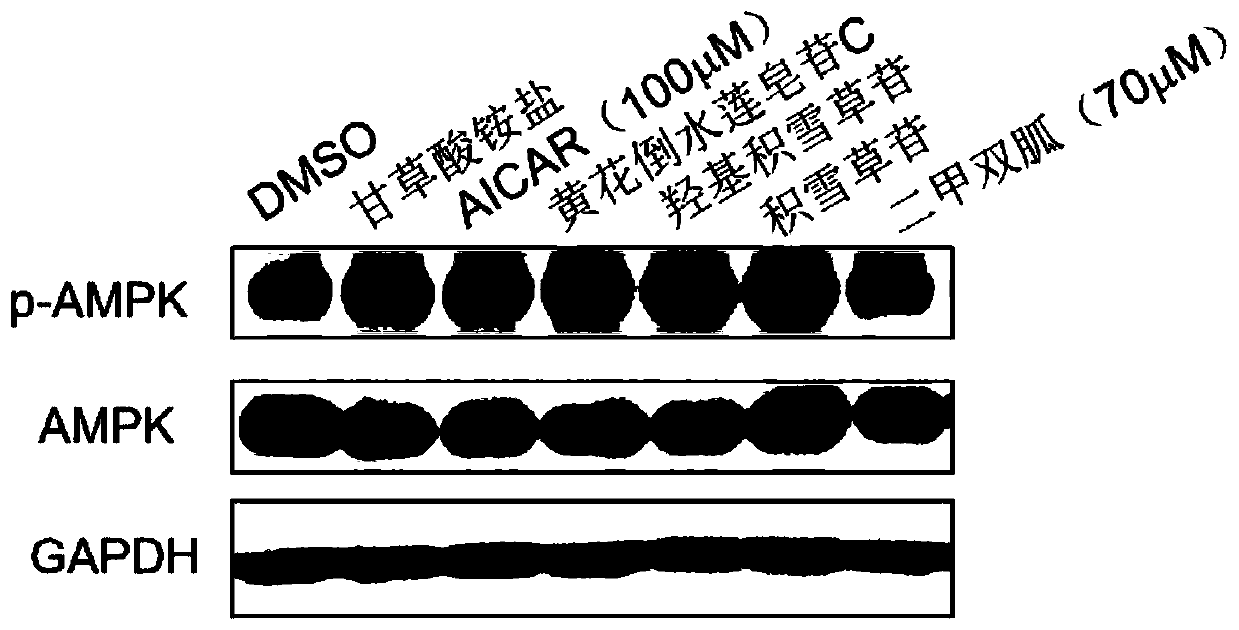
[0065] Agonist activity of pentacyclic triterpene saponins on AMPK in Huh-7 cells
[0066] The agonistic activity of compounds on AMPK in Huh-7 cells was detected by Western Blot method.
[0067] Cell culture conditions: Huh-7 cells: DMEM complete medium (containing 10% fetal bovine serum and 1% streptomycin / penicillin) in 5% CO 2 cultured in a 37°C incubator.
[0068]Antibodies: anti-AMPK (CST, 2532S); anti-pAMPK (CST, 2535S).
[0069] Western Blot experiment: To detect the effect of the compound on the phosphorylation level of AMPK in Huh-7 cells: cells with a proportion of living cells above 90% were used for the experiment. In 12-well plates, Huh-7 cells were plated at 250,000 per well, placed in 5% CO 2 After 12 hours, the original medium was discarded, and the complete medium containing pentacyclic triterpenoid saponins was added. The final concentration of the tested pentacyclic triterpene saponins was set to 10 μM, and the administration time was 12 hours. AICAR (...
Embodiment 2
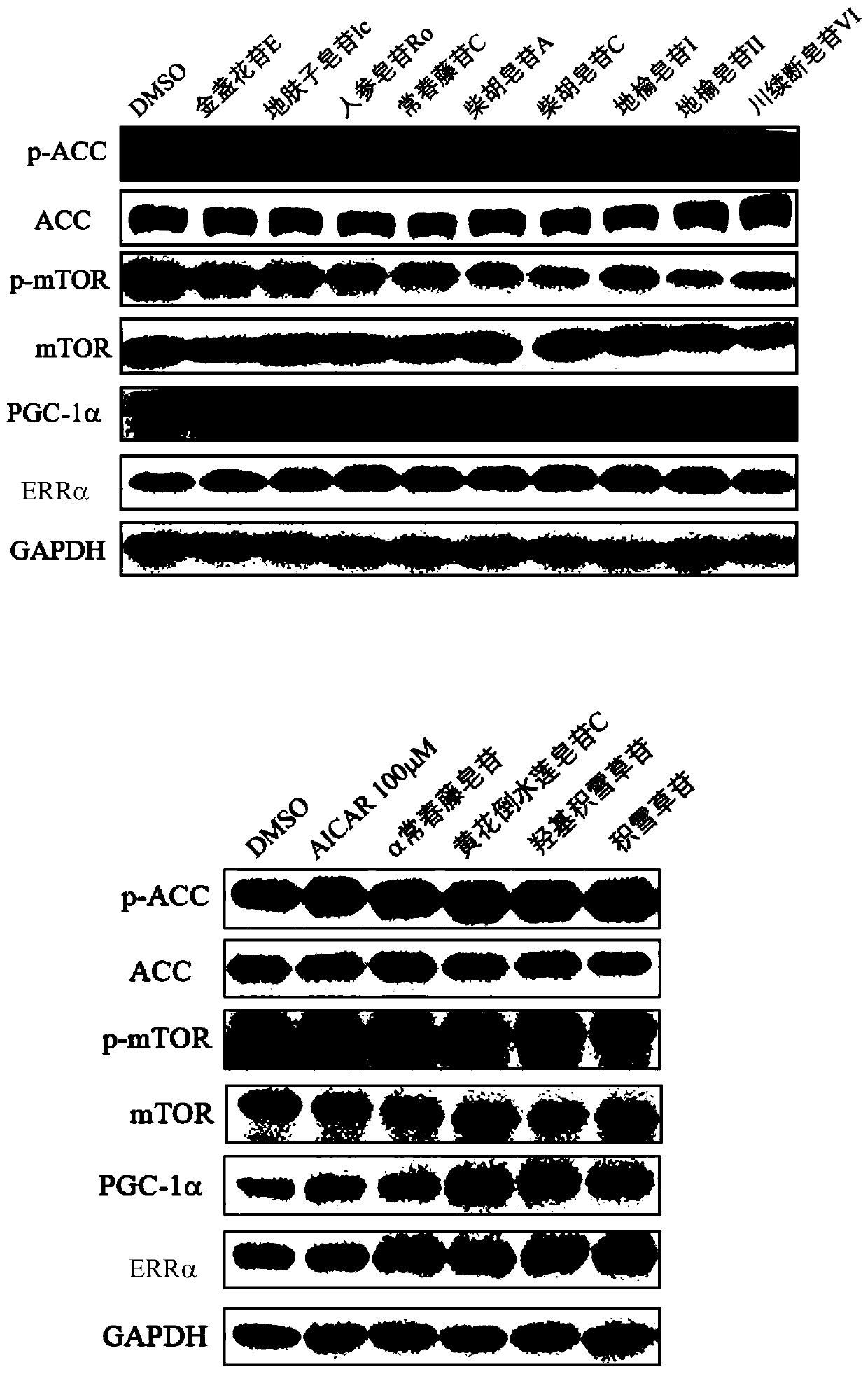
[0075] Effects of pentacyclic triterpene saponins on the downstream signaling pathway of AMPK in Huh-7 cells
[0076] Western Blot method was used to detect the effect of compounds on the downstream signaling pathway of AMPK in Huh-7 cells.
[0077] Cell culture conditions: Huh-7 cells: DMEM complete medium (containing 10% fetal bovine serum and 1% streptomycin / penicillin) in 5% CO 2 cultured in a 37°C incubator.
[0078] Antibodies: anti-ACC(CST,3676S); anti-pACC(CST,11818S); anti-mTOR(CST,2983S); anti-pmTOR(CST,5536S); anti-PGC1α(Abcam,ab54481); (Abcam, ab76228).
[0079] Western Blot experiment: detecting the effect of the compound on the downstream signaling pathway of AMPK in Huh-7 cells:
[0080] Cells with a live cell ratio above 90% were used for experiments. In 12-well plates, Huh-7 cells were plated at 250,000 per well, placed in 5% CO 2 After 12 hours, the original medium was discarded, and the complete medium containing pentacyclic triterpenoid saponins was ad...
Embodiment 3
[0083] Protective effect of ginsenoside Ro (compound of formula (I)) on high-fat diet (HFD)-induced NASH rat model.
[0084] Animals: 32 male SD rats, SPF grade, 6 weeks old, weighing 220 g, purchased from Beijing Weitong Lihua. All animals were maintained on a 12-h alternating circadian rhythm with free access to food and drink.
[0085] Instruments: animal weight scales; slicers; automatic biochemical analyzers; inverted microscopes
[0086] Reagents: Ginsenoside Ro(I) was purchased from Shanghai Boka Chemical Technology Co., Ltd.; obeticholic acid (OCA) was purchased from Jiangsu Vicare Pharmaceutical Technology Co., Ltd.; high-fat feed was purchased from Research Diets (D12492, 60kcal%); Standard feed was purchased from Research Diets (D12450B, 10 kcal%).
[0087] experiment procedure:
[0088] 1. Animal grouping and modeling
[0089] Rats were randomly divided into 4 groups according to body weight: control group (CHOW), high-fat diet model group (HFD), positive drug ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com