Individualized high purity hepatocellular carcinoma stem cells, methods and use of the same
A technology of hepatocellular carcinoma and cancer stem cells, which is applied in the field of personalized high-purity hepatocellular carcinoma stem cells, said stem cells and their uses, and can solve problems such as adverse effects, influence, and tumor resistance of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
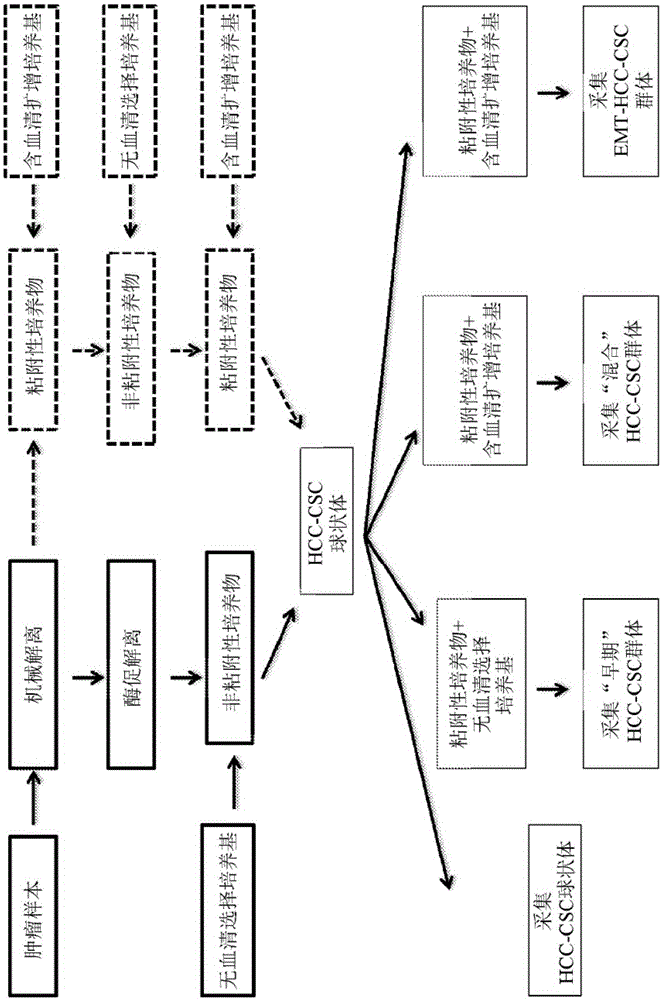
[0178] Isolation and Expansion of HCC Cancer Stem Cells from Needle Aspiration Biopsy
[0179] Hepatocellular carcinoma (HCC) tumor samples are histologically heterogeneous, consisting of more or less differentiated cancer cells as well as normal parenchymal, stromal, and vascular cells. The aim of the method presented here is to isolate and expand populations of cancer stem cells derived from smaller HCC samples. In addition, these cells are used to prepare autologous therapies for the treatment of HCC and the prevention of HCC recurrence.
[0180] Procedures and reagents are designed to maintain typical stem cells without maintaining the in vitro persistence / proliferation of differentiated hepatocytes, biliary epithelial cells, vascular endothelial cells, smooth muscle cells or fibroblasts. Typical components described in the literature, such as corticosteroids and serum, are omitted; instead, a basal medium formulation with specific ratios of amino acids and vitamins sup...
Embodiment 2
[0193] Generation of Loaded Dendritic Cell Compositions
[0194] The source of antigens is autologous tumor cells derived from continuously proliferating self-regenerative cells derived from fresh tumor tissue of the patient. These cells have the characteristics of cancer stem cells. At all times in the surgical and lesion setting, biopsies were handled with strict adherence to a sterile protocol to ensure that the specimens were sterile.
[0195] A pathologist obtains fresh tissue from a biopsy of a patient's tumor. Using a sterile scalpel and forceps, the samples were cut into 10 mm sections and transferred to transport tubes containing transport medium, processed promptly to avoid drying of the samples. Samples were shipped to the manufacturing facility by overnight courier within 48 days of surgical resection.
[0196] In the manufacturing facility, samples were dissociated into single cell suspensions in a clean room and placed in cell culture conditions designed to ...
Embodiment 3
[0201] Phase I trial of active-specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma
[0202] Because micrometastatic disease is undetectable at diagnosis, HCC is rarely cured with standard therapies. In addition, HCC is often associated with hepatitis B virus (HBV) infection, which renders patients ineligible for liver transplantation. Active specific immunotherapy (ASI) using autologous dendritic cells loaded with antigens derived from autologous tumor stem cells has been associated with promising long-term survival outcomes in patients with metastatic melanoma, although data from those trials Patients with HBV were excluded. This ASI approach deserves evaluation in other tumor types, including HCC. Surgical resection is part of standard therapy for many patients, thereby providing a tumor source for the generation of autologous tumor cell lines. Although A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com