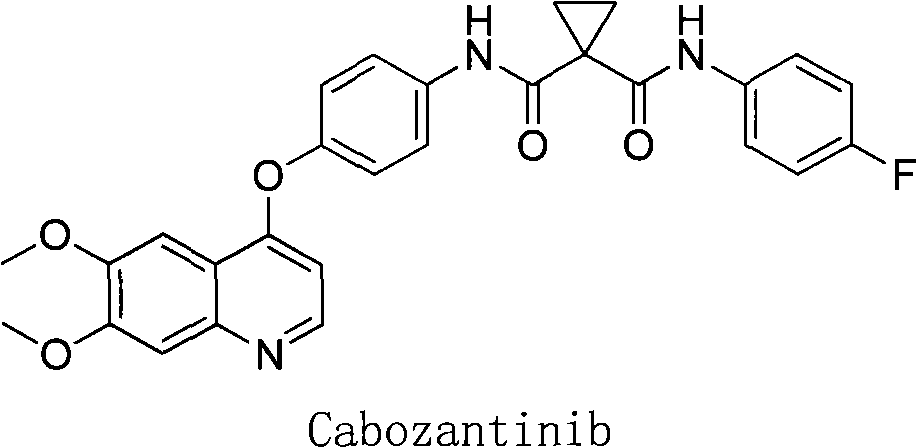
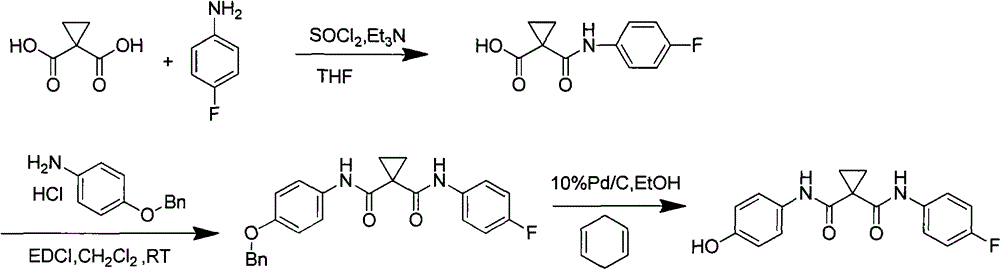
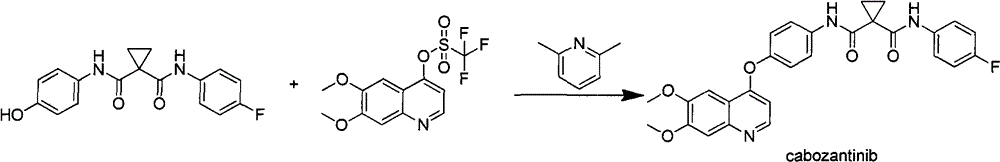
A kind of preparation method of tyrosine kinase inhibitor and its intermediate
An aminophenol and compound technology, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high price and cumbersomeness, and achieve the effects of simple operation, high yield and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0066] The preparation of embodiment 11-(ethoxycarbonyl) cyclopropane formic acid
[0067]
[0068] Add 200ml of ethanol and 30g (161mmol) of diethyl 1,1-cyclopropanedicarboxylate into a 500ml three-necked flask, cool to 10°C, add dropwise sodium hydroxide solution (NaOH6.4g, 161mmol; water 32ml) while stirring . After dropping, the mixture was reacted at room temperature for 3 hours. After the reaction was completed, the solvent was evaporated under reduced pressure to obtain a white solid. Add 200ml of water and 100ml of ethyl acetate to the solid, stir and separate the layers, and discard the organic phase. The aqueous layer was adjusted to pH 3-4 with 2mol / L hydrochloric acid, extracted with ethyl acetate (100ml×2), and the ethyl acetate layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a colorless translucent oily substance 20.0 g (78.5%).
Embodiment 21
[0069] The preparation of embodiment 21-(methoxycarbonyl) cyclopropanecarboxylic acid
[0070]
[0071] Using the method of Example 1, 1-(methoxycarbonyl)cyclopropanecarboxylic acid was prepared from 1,1-cyclopropanedicarboxylic acid dimethyl ester as a raw material.
Embodiment 31
[0072] Preparation of Example 31-(isopropoxycarbonyl)cyclopropanecarboxylic acid
[0073]
[0074] Using the method of Example 1, 1-(isopropoxycarbonyl)cyclopropanecarboxylic acid was prepared from 1,1-cyclopropanedicarboxylate diisopropyl ester as raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com