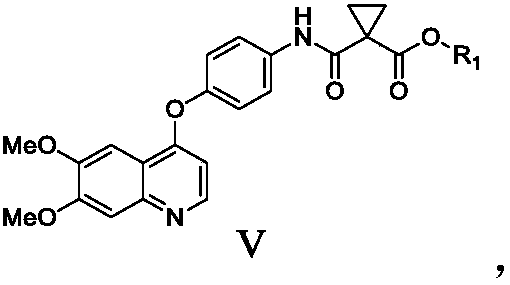
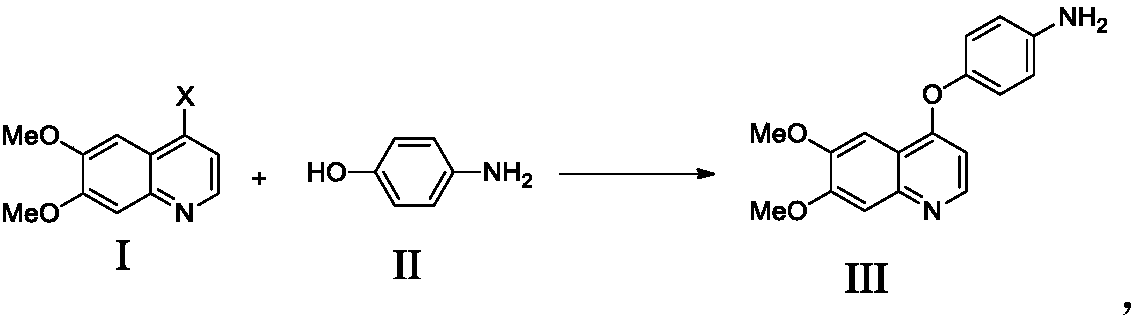
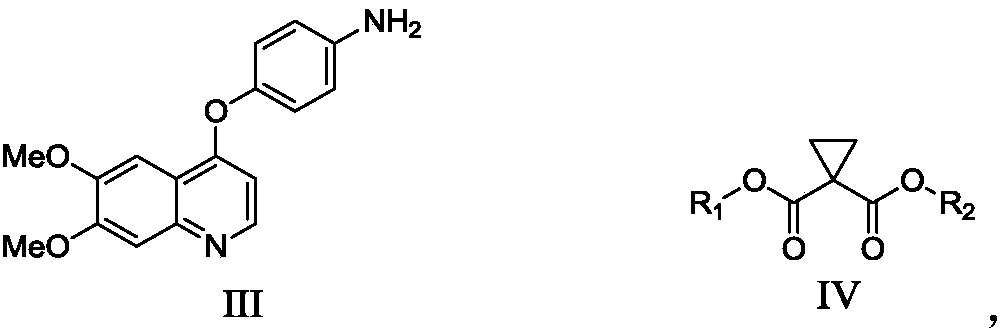Preparation method of Cabozantinib
A technology of cabozantinib and compounds, which is applied in the field of synthesis of cabozantinib and its intermediates, and can solve the problems of cumbersome operation, high cost, and low industrial production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of 4-((6,7-dimethoxy-4-yl)oxy)aniline
[0028]
[0029] Add 4-chloro-6,7-dimethoxyquinoline (10g, 0.045mol, 1.0eq.), 4-aminophenol (6.9g, 0.063mol, 1.4eq.) to 50mL N,N-dimethyl In dimethylacetamide, cool down to 0°C, slowly add a suspension of sodium tert-butoxide (6.1g, 0.063mol, 1.4eq.) and 50mL N,N-dimethylacetamide, after the addition is complete, heat up to 100°C , reacted for 5 hours, cooled the reaction liquid to 0°C, added 400 mL of purified water, stirred and crystallized for 15-16 hours. Stop stirring, filter, and wash the filter cake with 20 mL of purified water to obtain 11.2 g of 4-((6,7-dimethoxy-4-yl)oxy)aniline with a yield of 84.5% and a purity of 99.1%.
[0030] MS(ESI): m / z 297.20[M+H] + .
[0031] 1 H NMR (DMSO-d 6 ,400MHz): δ3.94(s,6H),5.19(s,2H),6.38(d,J=2.8Hz,1H),6.68(d,J=8.4Hz,2H),6.93(d,J= 8.8Hz, 2H), 7.37(s, 1H), 7.51(s, 1H), 8.43(d, J=5.2Hz, 1H).
Embodiment 2
[0032] Example 2 Preparation of 4-((6,7-dimethoxy-4-yl)oxy)aniline
[0033]
[0034] Add 4-chloro-6,7-dimethoxyquinoline (10g, 0.045mol, 1.0eq.), 4-aminophenol (6.9g, 0.063mol, 1.4eq.) to 50mL N,N-dimethyl In dimethylacetamide, cool down to 0°C, slowly add a suspension of sodium tert-butoxide (6.1g, 0.063mol, 1.4eq.) and 50mL N,N-dimethylacetamide, after the addition is complete, heat up to 110°C , reacted for 4 hours, cooled the reaction solution to 0°C, added 400 mL of purified water, stirred and crystallized for 15-16 hours. Stop stirring, filter, and wash the filter cake with 20 mL of purified water to obtain 11.5 g of 4-((6,7-dimethoxy-4-yl)oxy)aniline with a yield of 86.8% and a purity of 99.2%.
[0035] The mass spectrum and hydrogen spectrum data are basically consistent with Example 1.
Embodiment 3
[0036] Example 3 Preparation of 4-((6,7-dimethoxy-4-yl)oxy)aniline
[0037]
[0038] Add 4-chloro-6,7-dimethoxyquinoline (10g, 0.045mol, 1.0eq.), 4-aminophenol (6.9g, 0.063mol, 1.4eq.) to 50mL N,N-dimethyl In acetamide, cool down to 0°C, slowly add a suspension of potassium tert-butoxide (7.1g, 0.063mol, 1.4eq.) and 50mL N,N-dimethylacetamide, after the addition is complete, heat up to 100°C , reacted for 5 hours, cooled the reaction liquid to 0°C, added 400 mL of purified water, stirred and crystallized for 15-16 hours. Stop stirring, filter, and wash the filter cake with 20 mL of purified water to obtain 10.9 g of 4-((6,7-dimethoxy-4-yl)oxy)aniline, with a yield of 82.3% and a purity of 99.1%.
[0039] The mass spectrum and hydrogen spectrum data are basically consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com