Method for simultaneously determining cabozantinib analogue and related substances thereof
A technology of cabozantinib and analogs, applied in the field of drug analysis and detection, to achieve the effect of strong practicability, rapid time and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] [Example 1] Preparation of detection solution
[0105] 1.1 Preparation of impurity stock solution
[0106] Weigh about 10 mg of each impurity reference substance in the above table, put them in different 20ml measuring bottles, add acetonitrile to dissolve and dilute to the mark, shake well, and use as each impurity stock solution respectively.
[0107] 1.2 Preparation of the test solution
[0108] Accurately weigh about 50 mg of the cabozantinib analog bulk drug, put it in a 100ml measuring bottle, dissolve it with a solvent and dilute to the mark, and shake it up to obtain the test solution.
[0109] 1.3 Preparation of spiked test solution
[0110] Accurately weigh about 50 mg of the raw material drug of cabozantinib analogue, put it in a 100ml measuring bottle, add an appropriate amount of solvent to dissolve it, pipette 1ml each of the impurity A stock solution and the impurity B stock solution and place it in the measuring bottle, add solvent to dilute to scale,...
Embodiment 2
[0111] [Example 2] Screening of chromatographic conditions
[0112] 2.1 Selection of chromatographic column
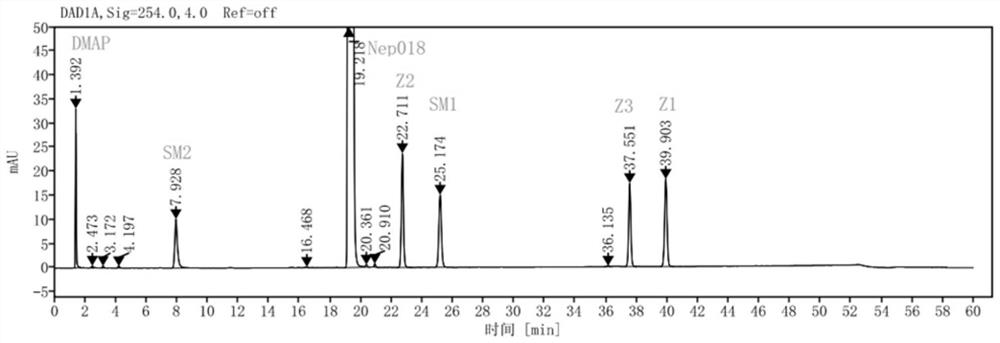
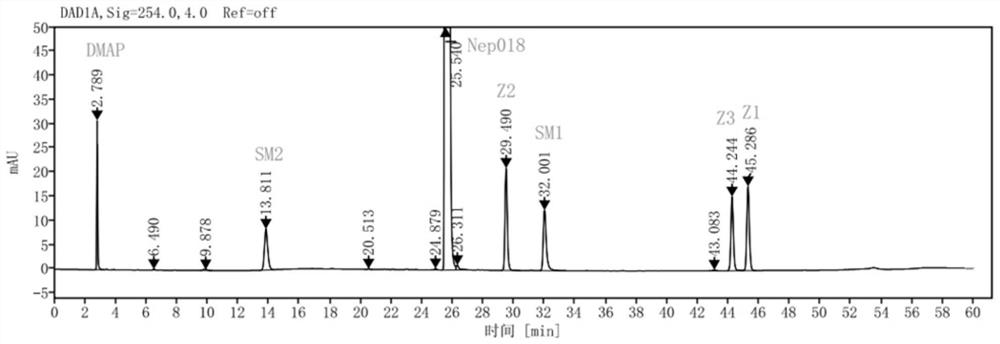
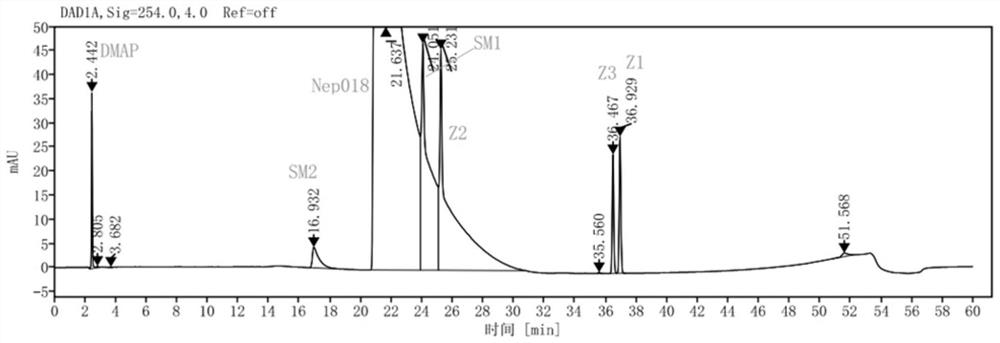
[0113] Get the need testing solution prepared by embodiment 1 and the need testing solution of standard addition, be divided into 5 groups, utilize the chromatographic column shown in table 1 respectively, detect according to following chromatographic conditions, obtain chromatogram respectively as attached Figure 1~5 shown.
[0114] Table 1 Columns
[0115]
[0116] Mobile phase A: phosphate buffered saline solution (take about 2.72 g of potassium dihydrogen phosphate, add 1000 ml of water to dissolve, adjust the pH value to 3.0 with 10% phosphoric acid);
[0117] Mobile phase B: acetonitrile;
[0118] Flow rate 1.0ml / min, column temperature 30°C, detection wavelength 254nm, follow the gradient elution procedure shown in Table 2:
[0119] Table 2 Gradient elution
[0120]
[0121] The retention time of the main peak, the number of theoretical plates, the t...
Embodiment 3
[0167] [Example 3] Forced degradation test
[0168] After the product is destroyed by high temperature, acid, alkali, oxidation, light and other severe conditions, the relevant substances are determined to check whether the selected chromatographic conditions can detect the possible degradation products of the product, as follows:
[0169] (1) Undestroyed test solution: take about 10 mg of this product, weigh it accurately, put it in a 20 ml measuring bottle, add 30% acetonitrile to dissolve and dilute to the mark, shake well, and filter to obtain.
[0170] (2) Preparation of test solution for high temperature destruction: Take about 10 mg of this product, weigh it accurately, put it in a 20 ml measuring bottle, add appropriate amount of 30% acetonitrile to dissolve, heat in a water bath at 90°C for 7 hours, take it out and let it cool, add 30% acetonitrile to dilute To the scale, shake well and filter, that is.
[0171] (3) Light damage the test solution: take about 10mg of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com