Anti-tumor drug cabozantinib impurity, preparation method thereof and application thereof
An anti-tumor drug, the technology of cabozantinib, which is applied in the field of biomedicine to achieve the effect of accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
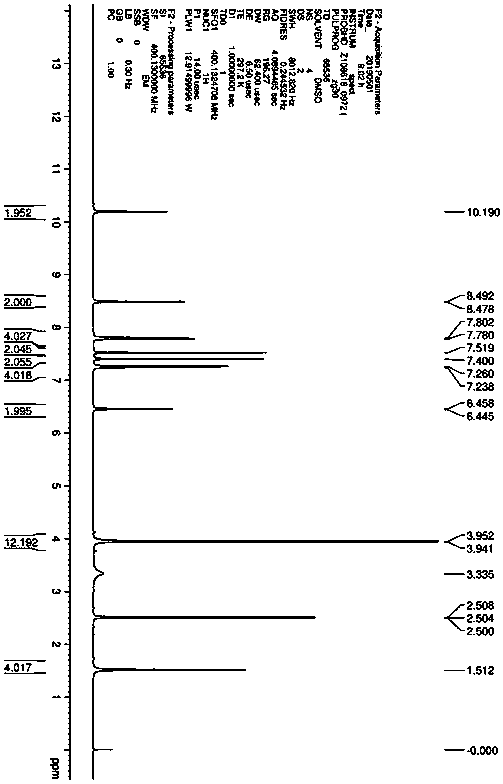
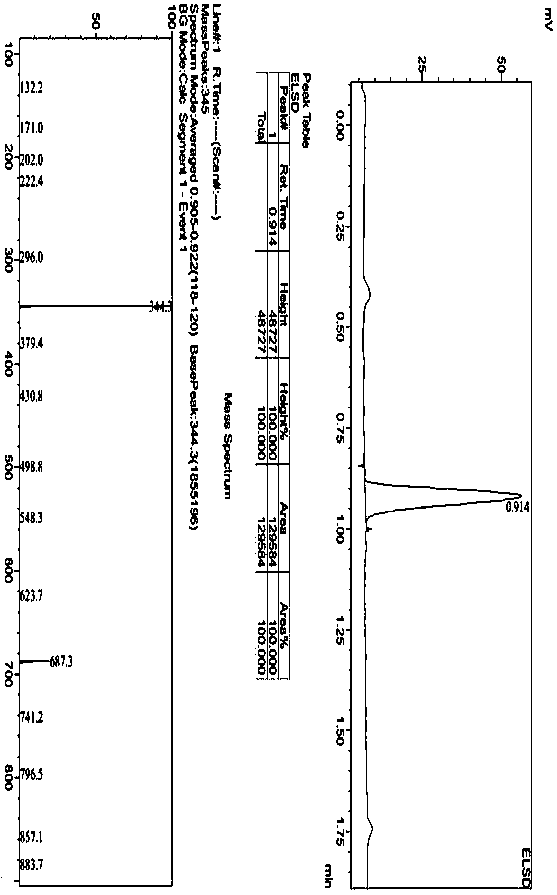
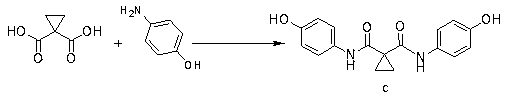
[0036] Add 1.0g of 1,1-cyclopropyldicarboxylic acid (7.68mmol) into the reaction flask, 20g of tetrahydrofuran, 3.1g of DIPEA, stir to dissolve, add 6.0g of HCTU and heat to 40°C, stir to activate 1,1-cyclopropane Add 2.4g (22mmol) of p-aminophenol after 1.5 hours of base dicarboxylic acid, and react at 20~40°C for 10~20 hours. After the reaction is completed, add 5wt% sodium carbonate solution to adjust the pH to 7~8, and then add 20g of purified water to crystallize , filtered and dried to obtain intermediate compound c 1.6g, yield 73%.
Embodiment 2
[0039]Add 20g of 1,1-cyclopropyldicarboxylic acid (153.7mmol) into the reaction flask, 140g of tetrahydrofuran, 60g of DIPEA, stir to dissolve, add 101.8g of HCTU and heat to 40°C, stir to activate 1,1-cyclopropyldicarboxylic acid After 1.5 hours of carboxylic acid, add 40g of p-aminophenol (366.5mmol), react at 20~40°C for 10~20 hours, after the reaction is completed, add 5wt% sodium carbonate solution to adjust the pH to 7~8, then add 80g of purified water to crystallize, filter After drying, 40.1 g of intermediate compound c was obtained, with a yield of 83%.
[0040] Take 30g of compound c, add it to 200g DMAC, add 32.3g of potassium tert-butoxide under stirring, stir until the temperature is stable, add 64.4g of 4-chloro-6,7-dimethoxyquinoline (compound d), and heat to React at 90-100°C for 8-10 hours, stop heating, add 300 g of purified water dropwise, let it cool down to room temperature for crystallization overnight, filter and wash to obtain the crude compound II. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com