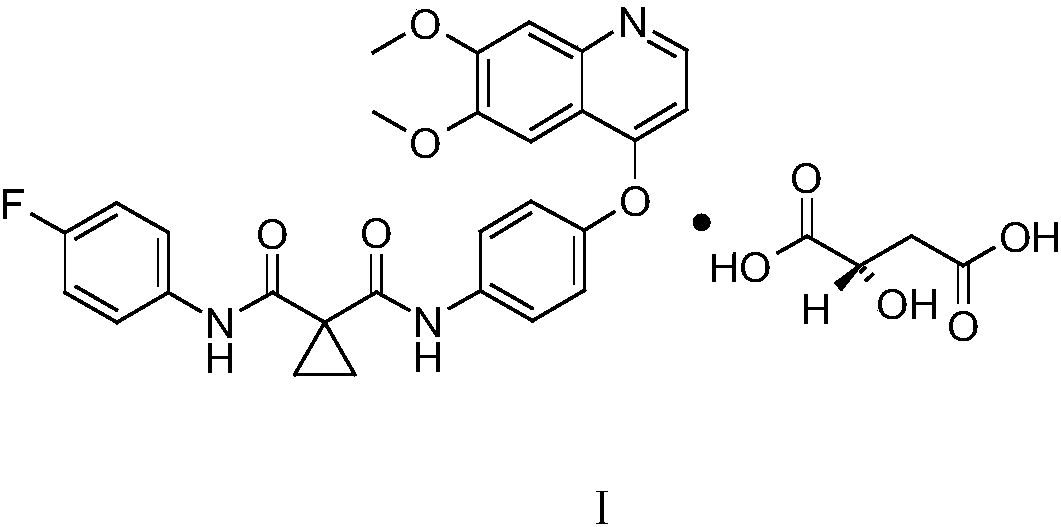
Synthetic method for cabozantinib
A technology of cabozantinib and its synthetic method, which is applied in the field of preparation of raw drug cabozantinib, can solve the problems of unfavorable environment and human health, unfavorable industrial production, etc., achieve good purity and yield, be beneficial to industrial production, and avoid Effect of acyl chloride reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
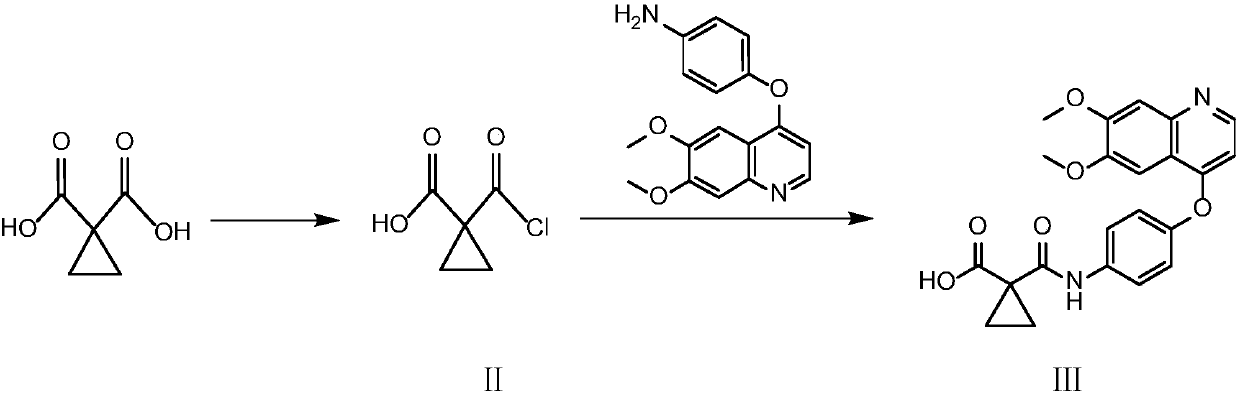
[0023] Preparation of Example 1N-{4-[(6,7-dimethoxy-4-quinolyl)oxy]phenyl}-1-formamide-cyclopropyl-1-carboxylic acid
[0024] Add triethylamine (48.5mL, 0.345mol) to a solution of 1,1-cyclopropyldicarboxylic acid (44.9g, 0.345mol) in tetrahydrofuran (350ml), and stir the solution at room temperature for 40 minutes under nitrogen protection, then add chlorine Sulfoxide (25mL, 0.344mol), LC / MS monitors the reaction, monitors the conversion rate of monoacyl chloride (monocarboxylate methyl ester is monitored after the reaction solution is quenched with methanol), after stirring at room temperature for 3 hours, successively add 4-[ (6,7-dimethoxy-4-quinoline) oxy]aniline (102g, 0.344mol) and tetrahydrofuran (150ml), continue to stir at room temperature for 16 hours, add ethyl acetate (1000ml) to dilute the reaction slurry , extracted with 1N sodium hydroxide solution. Filter the two-phase slurry thick liquid, adjust the pH of the water phase to 6 with hydrochloric acid and filter...
Embodiment 2
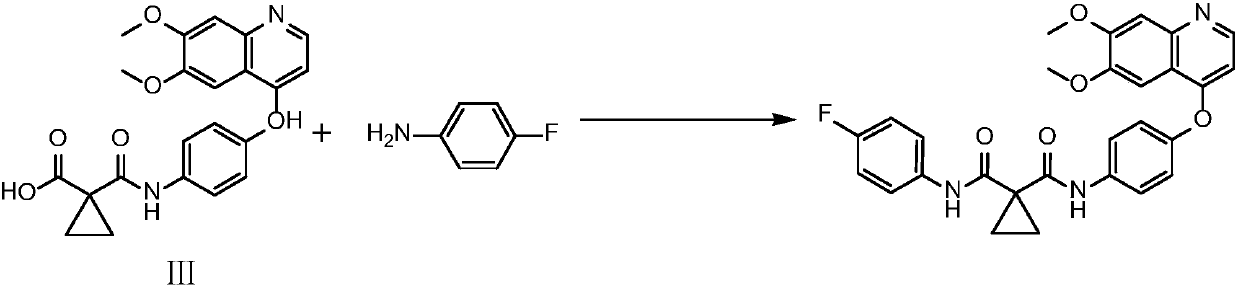
[0029] Add 1-[4-(6,7-dimethoxyquinoline-4-oxyl group) phenylcarbamoyl]-cyclopropyl-1-carboxylic acid (4.08g, 10mmol) in reaction flask, add N,N-Dimethylformamide was dissolved, then 1-hydroxybenzotriazole (HOBt, 2.43g, 18mmol), HB TU (4.55g, 12mmol) and N,N-diisopropylethylamine (1.55 g, 12mmol), after stirring evenly, add 4-fluoroaniline (1.11g, 10mmol), stir the reaction at room temperature overnight until the reaction is complete, add water (800ml) to dilute, stir and crystallize, filter, and filter the cake through 1N hydrochloric acid tetrahydrofuran solution respectively (800ml), 1N sodium hydroxide solution in tetrahydrofuran (800ml) and aqueous solution of tetrahydrofuran (800ml) were slurry washed and crystallized, filtered and dried to obtain 4.6g of cabozantinib base with a yield of 91.8% and a purity of 99.1%. MS: m / z 501[M+H] + . IR: 3442cm -1 、3244cm -1 、3071cm -1 、3021cm -1 、2959cm -1 、2836cm -1 、1672cm -1 、1642cm -1 、1618cm -1 、1589cm -1 、1567cm -1...
Embodiment 3
[0031] Add 1-[4-(6,7-dimethoxyquinoline-4-oxyl group) phenylcarbamoyl]-cyclopropyl-1-carboxylic acid (4.08g, 10mmol) in reaction flask, add N , N-dimethylformamide was dissolved, then added 1-hydroxybenzotriazole (HOBt, 2.43g, 18mmol), 2-(7-azobenzotriazole)-N,N,N',N '-Tetramethyluronium hexafluorophosphate (HATU, 4.56g, 12mmol) and N,N-diisopropylethylamine (1.55g, 12mmol), after stirring evenly, add 4-fluoroaniline (1.11g, 10mmol ), stirred at room temperature and reacted overnight until the reaction was complete, added water (800ml) for dilution, stirred and crystallized, filtered, and the filter cake was subjected to 1N hydrochloric acid tetrahydrofuran solution (800ml), 1N sodium hydroxide tetrahydrofuran solution (800ml) and tetrahydrofuran aqueous solution (800ml) respectively. After slurry washing and crystallization, filter and dry to obtain 4.3 g of cabozantinib base with a yield of 85.8% and a purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com