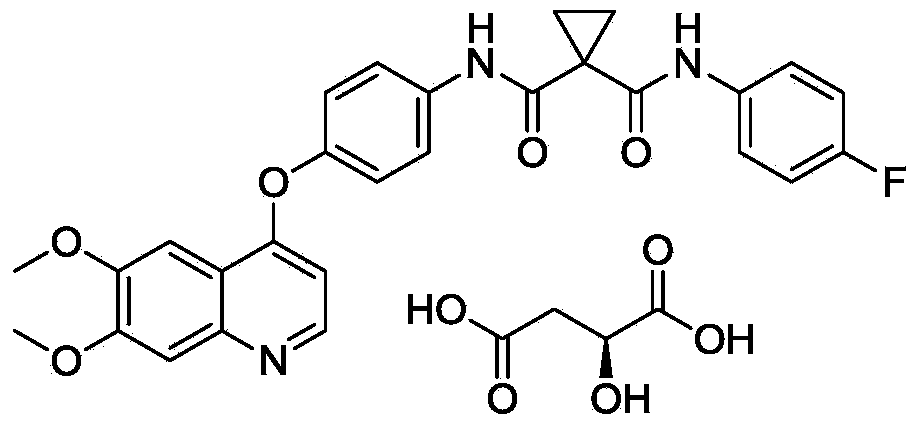
Cabozantinib dispersible tablets and preparation method thereof
A technology of dispersible tablets and weight percent, applied in the directions of heterocyclic compound active ingredients, drug combinations, pill delivery, etc., can solve the problems of low bioavailability, irritation to the body, and no acidifying agent, etc., to improve bioavailability. , increase compliance, reduce the effect of body stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Cabozantinib 250g
[0038] Lactose 120g
[0039] Cross-linked polyvinylpyrrolidone 25g
[0040] Carboxymethyl Starch Sodium 50g
[0041] Hypromellose 4g
[0042] Micronized silica gel 15g
[0044] water 60g
[0045] 95% ethanol 122g
[0046] Made in 1000 pieces.
[0047] Preparation:
[0048] Step 1: Pass the crushed Cabozantinib and pharmaceutical excipients through a 120-mesh sieve, swell the hydroxypropyl methylcellulose with hot water, stir until dissolved, and then add ethanol to make the ethanol concentration reach 70%.
[0049] Step 2: Mix Cabozantinib, lactose, crospovidone, and micronized silica gel evenly according to the prescription amount, and add the hypromellose solution described in Step 1 to make a soft material.
[0050] Step 3: Pass the soft material prepared in step 2 through a 18-mesh sieve to granulate, dry at 50°C, the water content is 2.8%, and granulate through an 18-mesh sieve.
[0051] Step 4: Mix the ...
Embodiment 2
[0055] Cabozantinib 250g
[0056] Lactose 80g
[0057] Cross-linked polyvinylpyrrolidone 14g
[0058] Carboxymethyl Starch Sodium 20g
[0059] Hypromellose 4g
[0060] Citric acid 100g
[0061] Micronized silica gel 15g
[0062] Magnesium Stearate 7.5g
[0063] water 250
[0064] 95% ethanol 125g
[0065] Made in 1000 pieces.
[0066] Preparation:
[0067] Step 1: Pass the crushed Cabozantinib and pharmaceutical excipients through a 120-mesh sieve, swell the hydroxypropyl methylcellulose with hot water, stir until dissolved, and then add ethanol to make the ethanol concentration reach 70%. Separately take 50 g of the above-mentioned hydroxypropyl methylcellulose solution, add 170 g of water, add citric acid and mix well.
[0068] Step 2: The prescribed amount of Cabozantinib and the citric acid solution in step 1 are embedded in the fluidized bed acid solution, and then mixed with the prescribed amount of lactose, cross-linked polyvinylpyrrolidone, sodium carboxymeth...
Embodiment 3
[0074] Cabozantinib 250g
[0075] Cross-linked polyvinylpyrrolidone 40g
[0076] Carboxymethyl Starch Sodium 40g
[0077] Hypromellose 3.5g
[0078] Citric acid 100g
[0079] Micronized silica gel 15g
[0080] Magnesium Stearate 7.5g
[0081] water 250
[0082] 95% ethanol 125g
[0083] Made in 1000 pieces.
[0084] The preparation method is the same as in Example 2.
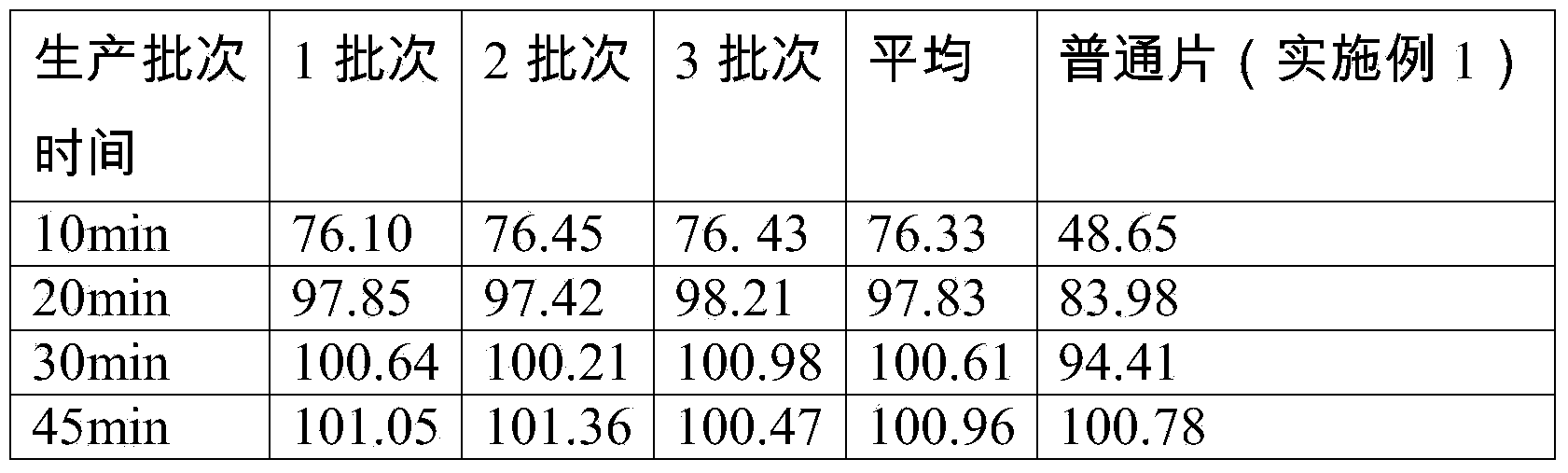
[0085] Implementation 3 increased the content of cross-linked polyvinylpyrrolidone and sodium starch glycolate, and the dispersibility of the dispersible tablets met the requirements of the 2010 edition of the Pharmacopoeia, and the dissolution rate was better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com