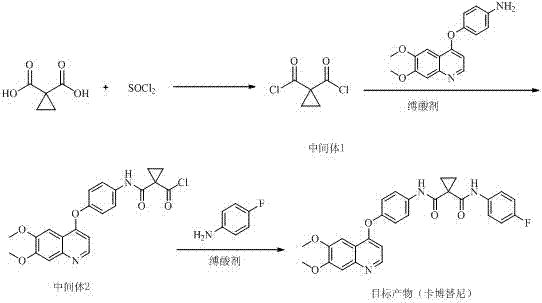
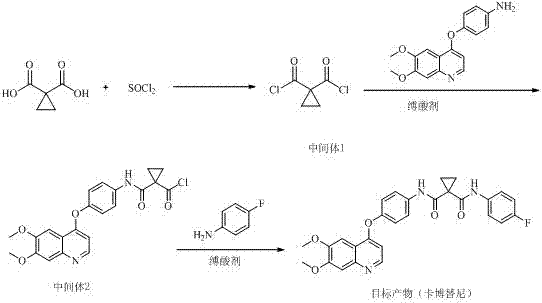
Method for preparing antitumor drug cabozantinib
A technology of cabozantinib and reaction, which is applied in the field of preparation of anti-tumor drug cabozantinib, can solve the problems of difficult monoamide product separation, increased material consumption, difficult control, etc., to simplify the operation process, simplify material consumption, Obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] Add 200g of 1,1-cyclopropyldicarboxylic acid into the reaction flask, add 560g of thionyl chloride, slowly heat to 20±2°C under stirring and react for 2 hours, stop the reaction and evaporate excess chloride under reduced pressure below 30°C Sulfoxide (about 200g), stop the distillation, add 1000g of tetrahydrofuran, 360g of triethylamine, stir and cool down to below 5°C. While stirring at this temperature, slowly add 440g of 4-[(6,7-dimethoxy-4-quinolyl)oxy]aniline (dissolved in 600g of tetrahydrofuran) below 5°C into the above reaction system dropwise, Add about 1 hour. Continue to stir the reaction for more than 5h. It was detected by TLC that the reaction of 4-[(6,7-dimethoxy-4-quinolyl)oxy]aniline was complete, and the temperature was raised to about 40°C, and 180g of p-fluoroaniline (diluted with 100g of tetrahydrofuran) was slowly added dropwise, about 1 hour added. Then raise the temperature to 50±5°C, continue to stir and react for more than 6 hours, change ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com