Cabozantinib preparation method
A technology of cabozantinib and organic solvents, applied in the field of preparation of cabozantinib, can solve problems such as poor economic benefits and environmental impact, low synthesis process yield, complicated operation, etc., and achieve simplified reaction process and post-treatment Process, optimized preparation process, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
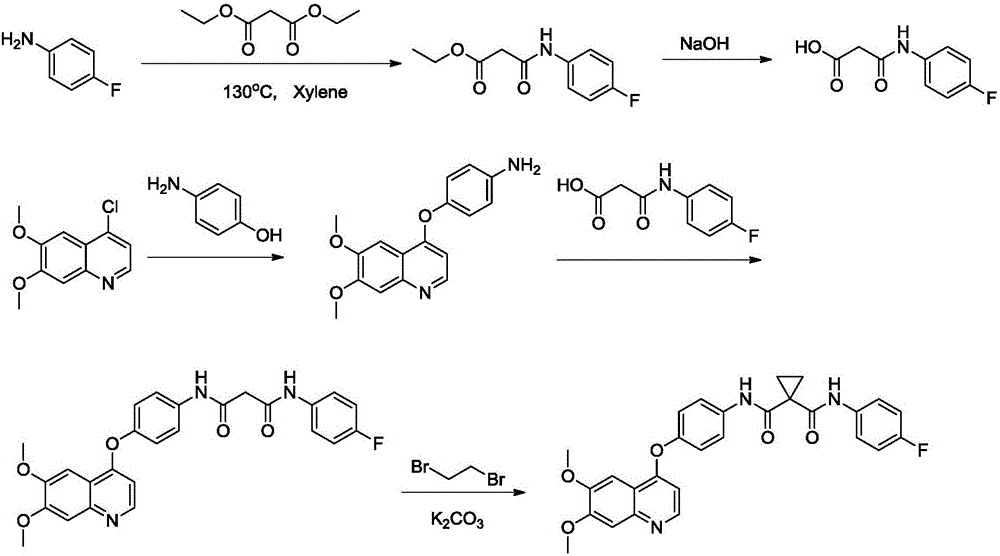
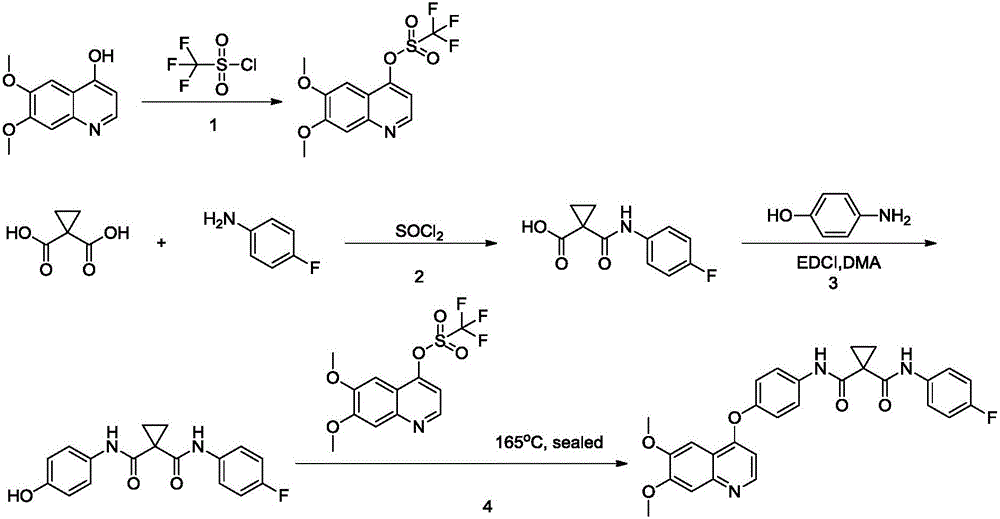
[0032] The first step: the synthesis of ethyl 3-(4-fluorophenylamino)-3-oxopropionate.
[0033] A solution of diethyl malonate (0.3mol, 48mL) in xylene (500ml) was added to a solution of 4-fluoroaniline (0.25mol, 23ml) in xylene (200ml), and the reaction mixture was refluxed for 1.5 hours. Spot the plate to monitor the completion of the reaction, the reaction solution was cooled to room temperature, n-hexane (200ml) was added to the reaction solution, filtered, the filter residue was washed with 1000ml of water, and the residue was recrystallized with ethanol to obtain a white solid (51.23g, yield 91%).
[0034] After testing, the nuclear magnetic spectrum is as follows, and it can be determined that the solid is ethyl 3-(4-fluorophenylamino)-3-oxopropionate.
[0035] 1 H NMR(CDCl3)1.30(t,3H),3.48(s,2H),4.20(q,2H),7.03(t,2H),7.61(dd,2H),9.40(s,1H)
[0036] The second step: the synthesis of 3-(4-fluorophenylamino)-3-oxopropionic acid.
Embodiment 2
[0054] The first step: the synthesis of ethyl 3-(4-fluorophenylamino)-3-oxopropionate.
[0055] A solution of diethyl malonate (0.3mol, 48mL) in toluene (500ml) was added into a solution of 4-fluoroaniline (0.25mol, 23ml) in toluene (200ml), and the reaction mixture was refluxed for 1.5 hours. Spot the plate to monitor the completion of the reaction, the reaction solution was cooled to room temperature, n-hexane (200ml) was added to the reaction solution, filtered, the filter residue was washed with 1000ml water, and the residue was recrystallized with ethanol to obtain a white solid 3-(4-fluorophenylamino)- Ethyl 3-oxopropanoate (52.1 g, yield 92.5%).
[0056] The second step: the synthesis of 3-(4-fluorophenylamino)-3-oxopropionic acid.
[0057] Lithium hydroxide solution (1N, 600ml) was added at 0°C in methanol (700ml) of ethyl 3-(4-fluorophenylamino)-3-oxopropionate (100g, 0.444mol), and the reaction solution was Stir at 20°C for 1 hour. The reaction solution was spin-d...
Embodiment 3
[0066] The first step: the synthesis of ethyl 3-(4-fluorophenylamino)-3-oxopropionate.
[0067] A solution of diethyl malonate (0.275mol, 44mL) in xylene (500ml) was added to a solution of 4-fluoroaniline (0.25mol, 23ml) in xylene (200ml), and the reaction mixture was refluxed for 1.5 hours. Spot the plate to monitor the completion of the reaction, the reaction solution was cooled to room temperature, n-hexane (200ml) was added to the reaction solution, filtered, the filter residue was washed with 1000ml water, and the residue was recrystallized with ethanol to obtain a white solid 3-(4-fluorophenylamino)- Ethyl 3-oxopropanoate (52.9 g, yield 93.96%).
[0068] The second step: the synthesis of 3-(4-fluorophenylamino)-3-oxopropionic acid.
[0069] Potassium hydroxide solution (1N, 600ml) was added at 0°C to ethyl 3-(4-fluorophenylamino)-3-oxopropionate (100g, 0.444mol) in tetrahydrofuran (700ml), and the reaction solution was Stir at 10°C for 1 hour. The reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com