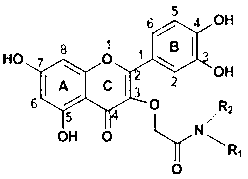
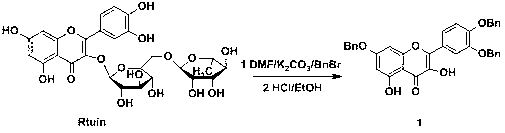Preparation method and use of quercetin amide derivative
A quercetin amide and derivative technology, applied in the field of medicinal chemistry, can solve the problems of low bioavailability, limitation of quercetin activity research and clinical application, poor solubility of quercetin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] 2 Preparation of compounds
[0041] 2.1 Synthesis of 3′,4′,7-O-tribenzylquercetin (1)
[0042] Place rutin in a vacuum oven at 80°C for 10 hours to remove crystal water, dissolve 4.88g (8 mmol) of rutin in 40mL N,N-dimethylformamide (DMF), and then add 3.86g (28 mmol) anhydrous K 2 CO 3, stirred at room temperature for 15 min, slowly added benzyl bromide 3.4 mL (28 mmol) dropwise under ice bath, and reacted at room temperature for 16 h. Adjust the pH to 6~7 with glacial acetic acid, add 200 mL of distilled water, and stir for 3 h until the solid precipitates and the water becomes clear. Discard the water layer, add 80 mL of ethanol to dissolve, add 18 mL of concentrated hydrochloric acid, reflux in water bath for 2 h, precipitate a large amount of yellow precipitate, filter, wash with water to obtain the crude product, and recrystallize with chloroform / methanol to obtain 3.72 g of pure product 1, yield 81.2% .
[0043] 1 H NMR (400 MHz, DMSO) δ 12.42 (s, 1H,5-...
Embodiment 1
[0049] Example 1: Preparation of quercetin-3-O-acetyl-isopropylamine (3-1)
[0050] Add 1.26g (2mmol) of compound 2 into a round bottom flask, add 40mL of anhydrous dichloromethane and stir to dissolve, add 454mg (2.2mmol) of DCC and 297mg (2.2mmol) of HOBt, and stir for 1h in an ice-salt bath. Dilute 130mg (2.2mmol) of isopropylamine with 20mL of anhydrous DCM, and slowly add it dropwise into the reaction flask with a constant pressure dropping funnel. After 20 minutes, gradually turn to room temperature and stir for 8-24 hours. After the reaction, put it in the refrigerator for half an hour. Most of the by-product DCU was removed by filtration, the filtrate was extracted, HOBt was easily soluble in water and removed, and the organic layer was dried overnight with anhydrous sodium sulfate. Filter, concentrate by rotary evaporation, add a small amount of acetone, a small amount of white granular solid precipitates, filter, and spin dry the filtrate. Recrystallization from ...
Embodiment 2
[0053] Embodiment 2: the synthesis of quercetin-3-O-acetyl-tert-butylamine (3-2)
[0054] The synthesis method is the same as 3-1. Compound 3-2 is a light yellow solid with a yield of 44.3%.
[0055] M.p: 274.8~276.6 ℃; 1 H NMR (400 MHz, DMSO) δ 12.49 (s, 1H,5-OH), 10.93 (s, 1H,7-OH), 9.84 (s, 1H, 4′-OH), 9.41 (s, 1H, 3 ′-OH), 7.77 (s, 1H,NH), 7.46 (dt, J = 8.3, 2.2 Hz, 2H, 2′-H, 6′-H), 6.92 (d, J = 8.3 Hz, 1H, 5 ′-H), 6.44 (d, J = 2.0 Hz, 1H, 8-H), 6.23 (d, J = 2.0 Hz, 1H, 6-H), 4.25 (s, 2H,COCH2), 1.29 (s, 9H.3×CH 3 ).
[0056] 13 C NMR (101 MHz, DMSO) δ 178.16, 167.47, 164.84, 161.61, 156.88, 156.54, 149.41, 145.86, 137.49, 121.46, 120.91, 116.28, 115.95, 104.43, 99.20, 94.22, 72.38, 50.68, 28.86.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com