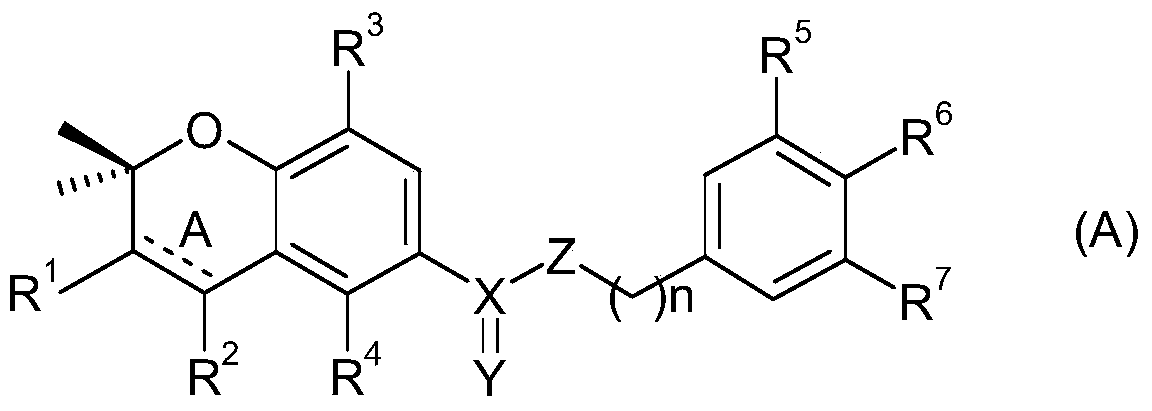
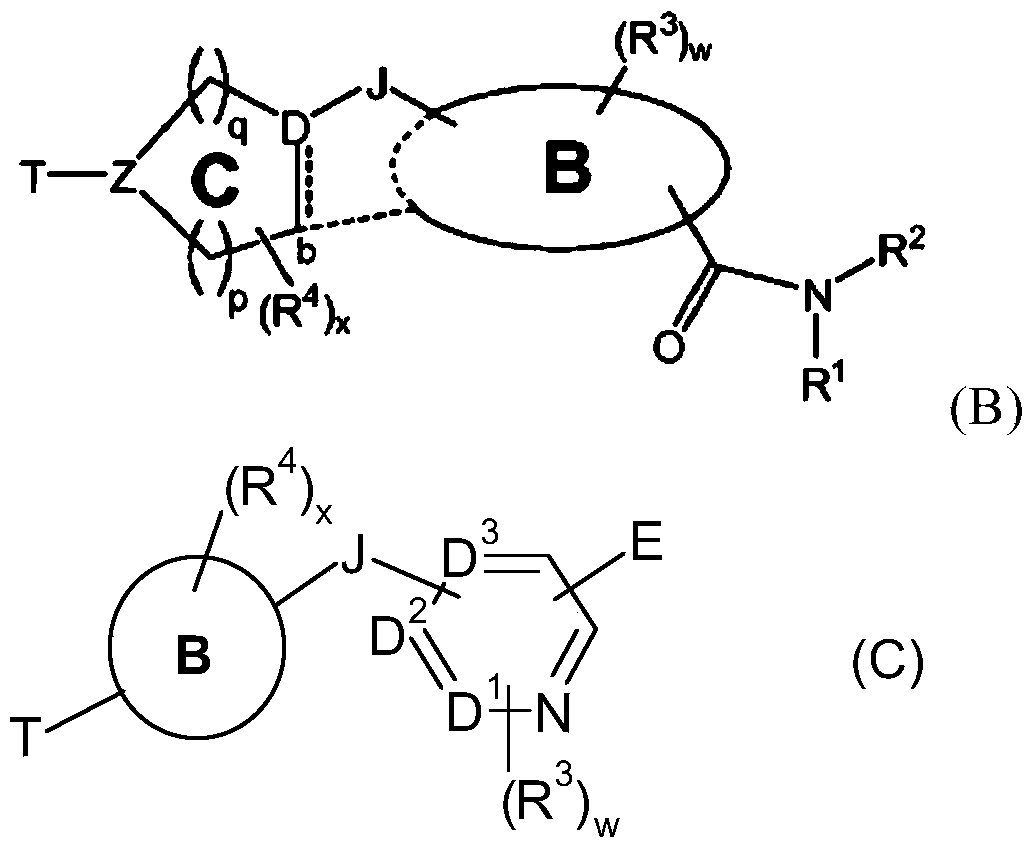
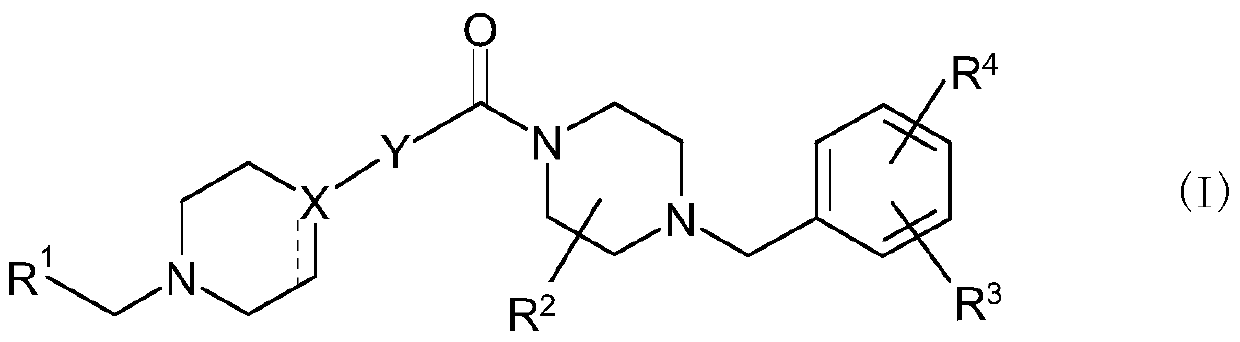
Bicyclic nitrogen-containing aromatic heterocyclic amides
A compound and composition technology, applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve problems such as the usefulness of cancer treatment without revelation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0338] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production methods of the raw material compounds are shown in the production examples, respectively. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, and the compound of formula (I) can also be obtained by a combination of these production methods, or it will be obvious to those skilled in the art method to manufacture.
[0339]In this specification, naming software such as ACD / Name (registered trademark, Advanced Chemistry Development, Inc.) may be used for naming compounds.
[0340] In addition, the concentration mol / l is expressed as M for the sake of convenience. For example, a 1M aqueous sodium hydroxide solution refers to ...
manufacture example 1
[0344] In 5-bromo-1H-benzimidazole-2-carboxylic acid (1.0g), 1-[4-(trifluoromethyl)benzyl]piperazine (1.0g), 1-hydroxybenzotriazole (840mg) and N,N-dimethylformamide (10ml: hereinafter abbreviated as DMF) mixture, add N-[3-(dimethylamino)propyl]-N'-ethylcarbodiimide hydrochloride ( 1.2 g) and stirred overnight at room temperature. Saturated aqueous sodium bicarbonate solution was added to the reaction mixture and stirred at room temperature for 1 hour, and the resulting solid was filtered off and dried under reduced pressure. The obtained solid was dissolved in a mixture of chloroform (100 ml) and ethanol (1 ml) under reflux with heating. The mixture was cooled to room temperature, then hexane (100ml) was added. The resulting solid was filtered off and dried under reduced pressure to give (5-bromo-1H-benzimidazol-2-yl){4-[4-(trifluoromethyl)benzyl]piperazine as a solid -1-yl}methanone (1.4 g).
manufacture example 2
[0346] A mixture of 2-chloroquinoline-6-carboxylic acid (500 mg), thionyl chloride (5 ml) and DMF (1 drop) was heated to reflux for 30 minutes, then allowed to cool to room temperature. To a mixture of the solid obtained by concentrating the reaction mixture under reduced pressure and dichloromethane were added 1-[4-(trifluoromethyl)benzyl]piperazine (620 mg) and triethylamine (340 µl) under ice-cooling , and stirred at room temperature for 2 hours. Saturated aqueous sodium bicarbonate solution was added to the reaction mixture, followed by extraction with chloroform. The organic layer was washed with a saturated aqueous sodium chloride solution, and then dried over anhydrous sodium sulfate. The desiccant was removed, and then the solvent was distilled off under reduced pressure. The obtained residue was purified by amino silica gel column chromatography (hexane-ethyl acetate) to obtain (2-chloroquinolin-6-yl){4-[4-(trifluoromethyl)benzyl Base]piperazin-1-yl}methanone (630 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com