Ursolic acid nitrogen heterocyclic ring structure modifiers with antitumor activity and preparation method for ursolic acid nitrogen heterocyclic ring structure modifiers
A technology of nitrogen-heterocycle and ursolic acid, applied in the field of ursolic acid nitrogen-containing heterocycle structure modification and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
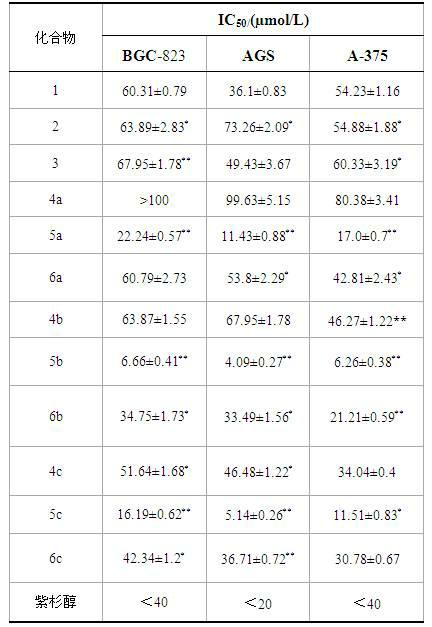
Examples
Embodiment 1
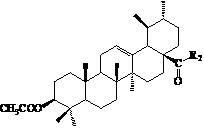
[0061] Preparation of 3-oxo-arbutane-12-ene-28-carboxylic acid (compound UA-2)
[0062] Preparation of PCC: In a 250mL three-neck flask, 50g CrO 3 Quickly add it to 92ml 6mol / L hydrochloric acid solution, and cool the homogeneous system in an ice bath after 5 minutes. After removing the ice bath, slowly drop 40 g of pyridine into the three-neck flask from the constant pressure funnel, and finish the dropping within at least 10 minutes. Cool the reaction system to 0 degrees Celsius again to obtain PCC as an orange-yellow solid. Filter it, dry it in vacuum for 2 hours, and seal it. refrigerated for later use;
[0063] Weigh 0.5g of UA, 0.7g of PCC, dissolve in 10mL of dichloromethane, stir at normal temperature and pressure, monitor the reaction process by TLC, end the reaction after 6-9 h, add water to stir and disperse, extract twice with chloroform, 25mL each time , Mix the organic phase, distill off the chloroform under reduced pressure, dissolve the residue with a little ...
Embodiment 2
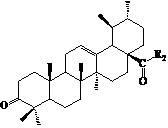
[0069] Preparation of 3-oxo-arbutane-12-ene-28--acyl chloride
[0070] Dissolve 250mg of UA-2 in 10mL of ether, add 0.25mL of oxalyl chloride dropwise in batches, stir and react at room temperature for 24-36 h, evaporate the solvent and the gas generated by the reaction, add 10mL of cyclohexane to dissolve to obtain white foamy solid, the cyclohexane was recovered under reduced pressure to take out the unreacted net oxalyl chloride, and recovered 2-3 times to obtain the crude product of 3-oxoursolic acid chloride intermediate, which was set aside.
Embodiment 3
[0072] Preparation of 3-oxo-arbutane-12-ene-28--acyl-(1-ethyl)piperazine (compound UA-4a)
[0073] Take 125ml of N-ethylpiperazine, dissolve it in 15mL of dichloromethane, adjust the pH of the solution to 8-9 with the acid-binding agent triethylamine, add 30mg of DMAP (4-dimethylaminopyridine) as a catalyst, and add the above The dichloromethane solution of the fresh 3-oxoursolic acid chloride intermediate is stirred and reacted at room temperature, and the reaction process is monitored by TLC. After 12-24 h, the reaction is terminated, the reaction solvent is evaporated under reduced pressure, and the residual solid is dissolved in dichloromethane. Wash with 1% dilute HCl solution to remove excess piperazine and triethylamine and other basic impurities, wash twice, discard the water layer, mix the organic phase, evaporate dichloromethane under reduced pressure, and wash the residual solid with water , dry at 50°C. The dried product was purified by silica gel column chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com