Ursolic acid diethanol amine derivative with anti-tumor activity and preparation method thereof
A technology of ursolic acid diethanol and amine derivatives, applied in the field of ursolic acid derivatives and their preparation, to achieve a strong tumor inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
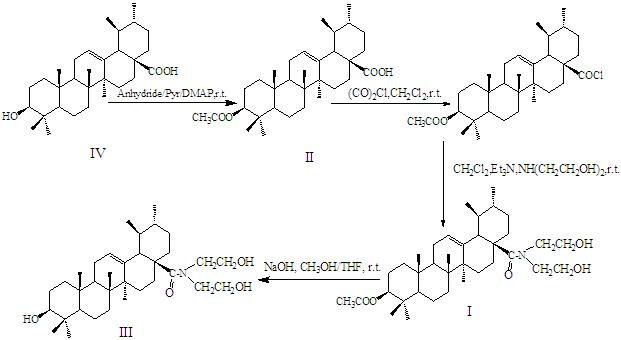
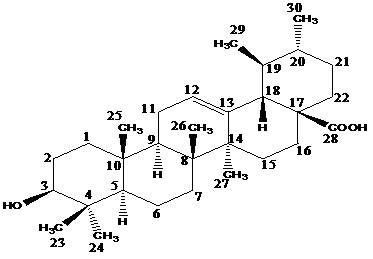
[0038] Preparation of 3-O-acetyl ursolic acid (compound II):
[0039] Weigh 0.7062 g of ursolic acid, add 30 ml of pyridine, stir to dissolve, add 1.8 ml of acetic anhydride dropwise, add a small amount of DMAP, stir at room temperature for 16 h, after the reaction, adjust the pH to 3-4 with 2 N HCl, evaporate Solvent, suction filtration, washing filter cake with water until neutral, drying at normal pressure 100°C, dry loading of the dried product, column chromatography purification (petroleum ether (60-90°C): ethyl acetate = 3:1), Dissolve in absolute ethanol, cool and recrystallize to obtain 3-Oacetyl ursolic acid.
[0040] Properties: white powder; yield: 86.17%; mp: 286-288°C.
[0041] IR Data (KBr, cm -1 ) and its attribution: υ: 3293 (O-H stretching vibration), 2927 (C-H stretching vibration), 1736 (s, C=O stretching vibration), 1370 (UA A region characteristic absorption), 1245 (s, C-O-C asymmetric stretching vibration ) cm -1 .
[0042] ESI-MS : m / z 497.5 [M-H]...
Embodiment 2
[0045] Preparation of N-[3β-acetoxy-arbutan-12-en-28-yl]-aminodiethanol (compound I):
[0046] Weigh 0.1954 g 3-O-acetyl ursolic acid and dissolve in 20 ml CH 2 Cl 2 0.19 ml of oxalyl chloride was added dropwise in batches, stirred at room temperature for 36 h, and the solvent and gas generated by the reaction were evaporated to obtain the crude product of 3-O-acetyl UA acid chloride intermediate, which was directly used for the next reaction. Add 20 mL of CH to the above intermediate 2 Cl 2 , triethylamine to adjust the pH of the solution to 8-9, add 0.19 ml of diethanolamine, stir and react at room temperature for 3 h, after the reaction, add 10 ml of distilled water to the reaction solution, adjust the pH to 3-4 with 2N HCl, filter, and wash the filter cake with water to The filtrate was pH neutral and dried. Purified by column chromatography (petroleum ether (60-90°C): ethyl acetate = 1:3), dissolved in absolute ethanol, cooled and recrystallized to obtain N-[3β-acetox...
Embodiment 3
[0052] Preparation of N-[3β-hydroxy-arbutane-12-en-28-yl]-aminodiethanol (compound III):
[0053] Weigh 0.5859g of N-[3β-acetoxy-arbutane-12-en-28-yl]-aminodiethanol, dissolve it in 20ml CH 3 OH-THF (1:1.5 v:v), add 2.0ml 4N NaOH, stir at room temperature for 3.5 h, the reaction is complete, add an appropriate amount of water to the mixture, adjust the pH to 3-4 with 2 N HCl, evaporate under reduced pressure Solvent, washed with water and precipitated to neutral, purified by column chromatography (petroleum ether (60-90°C): ethyl acetate = 1:3), dissolved in absolute ethanol, cooled and recrystallized to obtain N-[3β-hydroxy-benzyl Fran-12-en-28-yl]-aminodiethanol.
[0054] Properties: white powder; yield: 89.37%; mp: 270-272°C.
[0055] IR data (KBr, cm -1 ) and its attribution: υ: 3399 (-OH stretching vibration), 2926 (C-H stretching vibration), 1606 (s, C=O stretching vibration, amide I peak), 1408 (UA A region characteristic absorption) cm -1 .
[0056] ESI-MS : m / z...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com