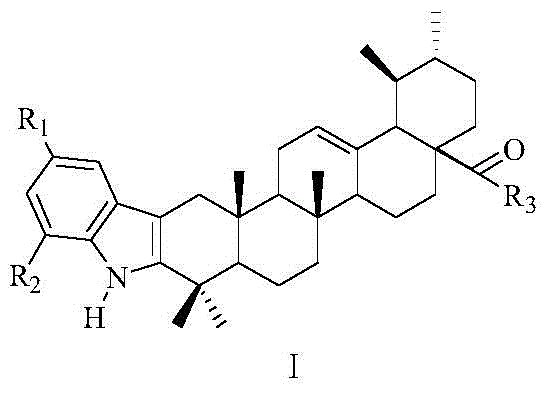
A class of indole ursolic acid derivatives, preparation method and use thereof
A technology of indole derivatives and ursolic acid, applied in the field of preparation of indole derivatives of ursolic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
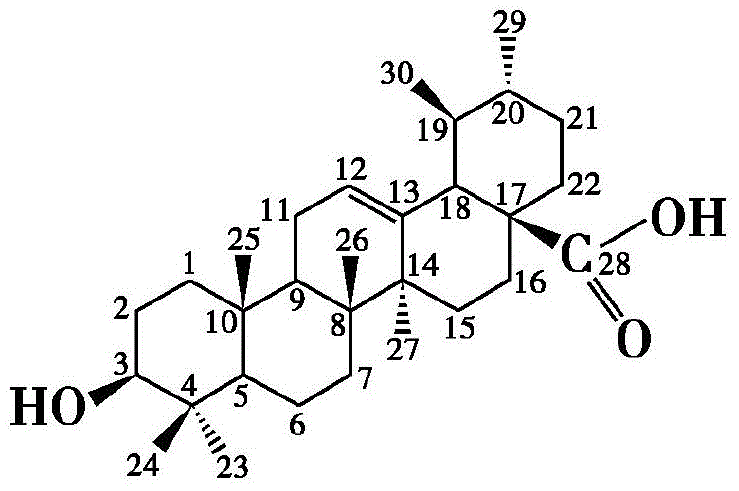
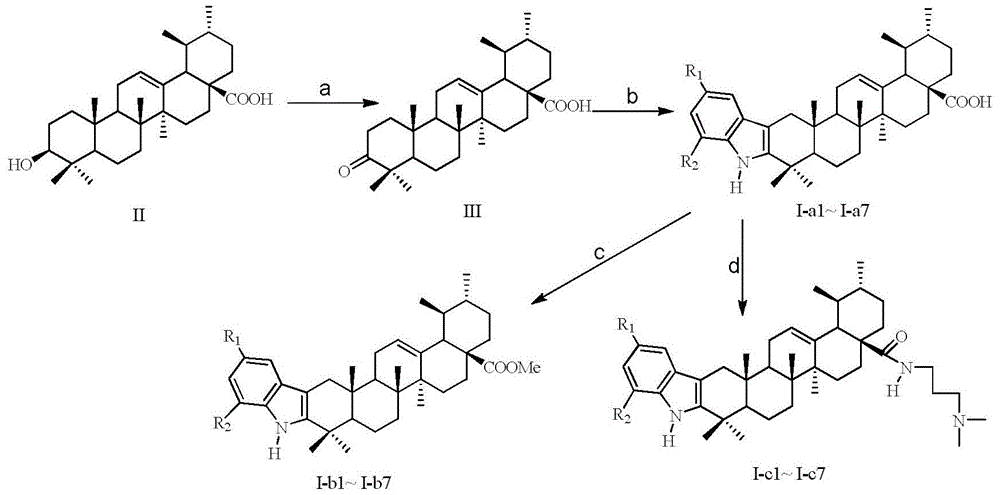
[0022] The synthesis of embodiment 1 oxidized ursolic acid (III)
[0023] Ursolic acid (1g, 2mmol) was dissolved in 150ml of acetone, and the reaction was placed in ice water and stirred for 15min, then slowly added Jones reagent dropwise (26.72g of chromium trioxide was dissolved in 23ml of concentrated sulfuric acid, then diluted to 100ml with water. obtained) 1ml, the reaction was stirred at room temperature for 5h, 45ml of isopropanol was added and stirred for 30min, and the precipitate was filtered off, and the solution was concentrated under reduced pressure to remove the solvent. Recrystallization from methanol gave white crystals of 3-oxyursolic acid III (0.56 g, 64%).
[0024] m.p.276-278°C; 1 H NMR (CDCl 3 ,300MHz):δ0.88(s,3H),0.91(d,J=6.3Hz,3H),0.96(s,3H),0.95(m,2H),0.99(d,J=6.0Hz,3H) ,1.07(s,3H),1.11(s,3H),1.13(s,3H),1.36(m,5H),1.52(m,5H),1.64(td,J=14.3,7.0Hz,1H), 1.76(dd,J=13.6,7.0Hz,1H),1.94(m,2H),2.24(d,J=11.2Hz,1H),5.31(m,1H); IR(KBr,cm -1 ):2930,1718,1657...
Embodiment 2
[0025] Embodiment 2 The synthesis of the phenylhydrazine hydrochloride of p-methyl substituent
[0026] Dissolve p-methylaniline (0.32g, 3mmol) in 3ml of 20% hydrochloric acid, dissolve sodium nitrite (0.28g, 4mmol) in 0.7ml of water, and slowly add it dropwise to the above solution under ice-bath conditions. After stirring and reacting for 1 h under the conditions, the tin protochloride (1.354 g, 6 mmol) solution dissolved in 1.8 ml of 35% hydrochloric acid was slowly dripped into the above reaction for 2 h, and the filtrate was removed by suction filtration to obtain solid phenylhydrazine hydrochloride ( 0.34 g, 70%).
Embodiment 3
[0027] Embodiment 3 The synthesis of the phenylhydrazine hydrochloride of p-methoxy substituent
[0028] P-methoxyaniline (0.37g, 3mmol) was dissolved in 3ml of 20% hydrochloric acid, sodium nitrite (0.28g, 4mmol) was dissolved in 0.7ml of water and slowly added dropwise to the above solution under ice-bath conditions, and the mixture was ice After stirring and reacting for 1 h under bath conditions, slowly drop the tin protochloride (1.354 g, 6 mmol) solution dissolved in 1.8 ml of 35% hydrochloric acid into the above reaction for 2 h, remove the filtrate by suction filtration to obtain solid phenylhydrazine hydrochloride (0.38 g, 69%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com