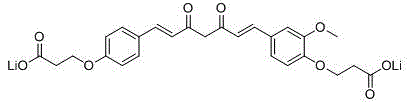
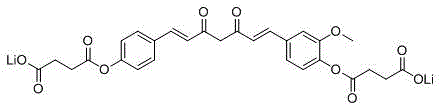
Water-soluble monodemethoxycurcumin derivative, and preparation method and use of water-soluble derivative
A technology of mono-demethoxy curcumin and methoxy curcumin carboxylic acid, applied in the field of mono-demethoxy curcumin water-soluble derivatives and preparation thereof, can solve the problem of insufficient activity, low bioavailability, water-soluble Sexual problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1. Preparation of 3-methoxy-4-(2-carboxyacetoxy)benzaldehyde
[0066]
[0067] Dissolve 5g of vanillin in 100mL of acetone, then add 6.8g of malonic acid to reflux, add 0.5mL of concentrated sulfuric acid during the reflux process, cool overnight, precipitate a solid, and recrystallize with methanol to obtain 5.89g of light yellow crystals, the yield 75.1%. 1 H NMR (CDCl 3 , 300 MHz) δ: 9.87 (s, 1H), 7,63 (m, 1H), 7.37 (dd, 1H), 7.34 (dd, 1H), 3.89 (s, 3H), 3.16 (s, 2H); ESI-MS m / z 238.1 [M+H] + .
Embodiment 2
[0068] Example 2. 3-methoxy-4-(4-carboxypropionyloxy)benzaldehyde
[0069]
[0070] Dissolve 5 g of vanillin in 50 mL of dichloromethane, add 4 g of succinic anhydride and a catalytic amount of DMAP, stir at room temperature for 36 hours, then add 100 mL of dichloromethane, wash the organic layer three times with saturated saline, and dry over anhydrous sodium sulfate. The organic layer was spin-dried, and recrystallized from methanol to obtain 5.77 g of light yellow crystals, with a yield of 69.6%. 1 H NMR (CDCl 3 , 300 MHz) δ: 9.89 (s, 1H), 7,62 (m, 1H), 7.39 (dd, 1H), 7.33 (dd, 1H), 3.87 (s, 3H), 3.26 (t, 2H), 3.15 (t, 2H); ESI-MS m / z 253.2 [M+H] + .
Embodiment 3
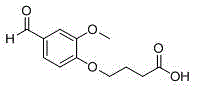
[0071] Example 3. 4-(4-formyl-2-methoxyphenoxy)butanoic acid
[0072]
[0073] Dissolve 3g of vanillin in 30mL of anhydrous acetone, add 5g of anhydrous potassium carbonate, slowly add 3.85g of ethyl bromobutyrate dropwise at room temperature, then overnight at room temperature, filter, spin dry acetone, add 30mL of methanol to dissolve, add 10% Sodium hydroxide solution 20mL, hydrolyzed overnight, adding 10% hydrochloric acid to adjust the pH to acidic, extracted with dichloromethane, combined the organic layer, dried over anhydrous sodium sulfate, spin-dried the organic layer, recrystallized from methanol to obtain 2.11g of a light yellow solid, the yield 45%. 1 H NMR (CDCl 3 , 300 MHz) δ: 9.84 (s, 1H), 7.72 (m, 1H), 7.51 (dd, 1H), 7.39 (dd, 1H), 3.92 (t, 2H), 3.88 (s, 3H), 2.26 ( t, 2H), 2.03 (m, 2H); ESI-MS m / z 238.4 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com