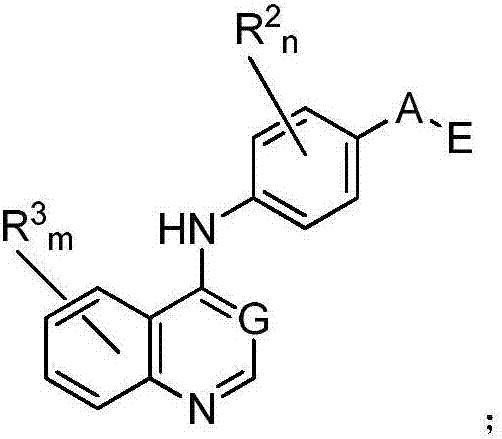
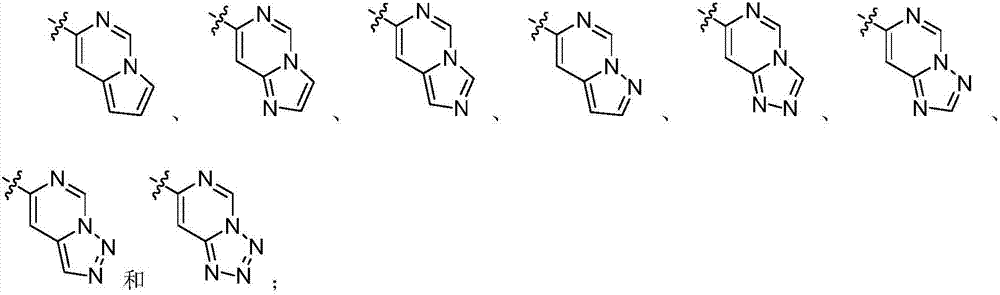
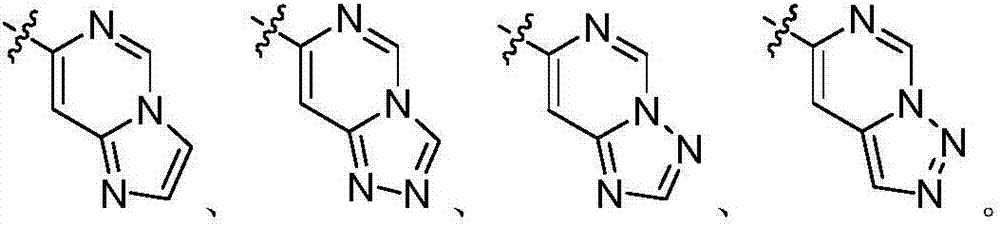
Nitrogenous heterocyclic compound, preparation method, intermediates, composition and application
一种氮杂环化合物、杂环基的技术,应用在含氮杂环化合物领域,能够解决毒副作用多、ErbB2选择性低、安全性低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0306]
[0307] N 4 -(4-([1,2,4]triazolo[1,5-c]pyrimidin-7-yloxy)-3-methylphenyl)-N 6 Synthesis of -(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine
[0308] Step A: Preparation of (E)-N'-(2-cyano-4-nitrophenyl)-N,N-dimethylformamidine: 2-amino-5-nitrobenzyl nitrile (11.5 g, 2.91mmol) was dissolved in N,N-dimethylformamide dimethyl acetal (40mL), heated to 100°C and stirred for 2 hours. After the reaction was completed, the solvent was directly evaporated to dryness under reduced pressure to obtain 15.1 g of light yellow solid, which was directly used in the next reaction.
[0309] Step B: Preparation of (E)-N'-(4-amino-2-cyanophenyl)-N,N-dimethylformamidine: (E)-N'-(2-cyano-4 -Nitrophenyl)-N,N-dimethylformamidine (1.0g, 4.58mmol) was dissolved in methanol:cyclohexene (5:1) mixed solvent (60mL), palladium carbon (10%, 100mg ), reacted under the protection of a hydrogen balloon, heated to 80 degrees and stirred for 18 hours. After the reaction was completed,...
Embodiment 3
[0316]
[0317] N 6 -(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N 4 Synthesis of -(4-(imidazo[1,2-c]pyrimidin-7-yloxy)-3-methylphenyl)-4,6-diaminoquinazoline
[0318] Step A: Preparation of 4-chloro-6-(2-methyl-4-nitrophenoxy)pyrimidine: Dissolve 4,6-dichloropyrimidine (1.5 g, 10.07 mmol) in 30 mL of N.N-dimethyl Add 2-methyl-4-nitrophenol (1.5g, 9.81mmol) and solid potassium carbonate (1.5g, 10.87mmol) to formamide, heat to 80°C and stir overnight. After the reaction was completed, 40 mL of ethyl acetate was added, stirred, filtered, and the filtrate was evaporated to dryness under reduced pressure. The residue was separated by column chromatography to obtain 2.3 g of a light yellow solid, with a yield of 88.46%.
[0319] Step B: Preparation of 6-(2-methyl-4-nitrophenoxy)-4-aminopyrimidine: 4-chloro-6-(2-methyl-4-nitrophenoxy)pyrimidine ( 1.2g, 4.51mmol) was dissolved in 20mL of ethanol and 60mL of ammonia solution, sealed tube and stirred at 120°C for 18 hours. After the r...
Embodiment 4
[0325]
[0326] N 4 -(4-([1,2,4]-triazolo[4,3-c]pyrimidin-7-yloxy)-3-toluene)-N 6 Synthesis of -(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-4,6-diaminoquinazoline
[0327] Step A: Preparation of 2-chloro-6-hydrazinopyrimidine: 2,6-dichloropyrimidine (25g, 167.81mmol) was dissolved in 350mL isopropanol, and hydrazine hydrate (29.5g, 503.44 mmol, 85%), exothermic during the dropwise addition and a white solid precipitated, and stirred at room temperature for 1 hour after the addition. The solvent was removed under reduced pressure, the residue was stirred with water (50 mL) for 30 minutes, filtered, the filter cake was washed with water, and dried to obtain 22.4 g of a white solid with a yield of 92.3%.
[0328] Step B: Preparation of 7-chloro-[1,2,4]triazolo[4,3-c]pyrimidine: 2-chloro-6-hydrazinopyrimidine (21 g, 145.27 mmol) was dispersed in 210 mL of trimethylorthoformate The ester was stirred overnight at 60°C, and the reaction liquid became clear. Add p-toluenesulfonic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com