Aminopeptidase N inhibitor, preparation method and application
A technology for aminopeptidase and inhibitors, applied in the field of aminopeptidase N inhibitors and preparation, capable of solving problems such as limited sources and high cost of total synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Aminopeptidase N inhibitor, chemical name: ( S )-4-Methyl-2-(3-naphthyl-1-ylmethyl-ureido)-pentanoylhydroxylamine. The structural formula of compound (I) is as follows:
[0029]
[0030] The preparation method, reaction steps and reaction formula of the compound are as follows:
[0031] It is the condensation of naphthyl methyl isocyanate and L-leucine methyl ester to form the corresponding urea-based intermediate, and the methyl ester is partially converted into hydroxamic acid to obtain compound (I). The reaction formula is as follows: Synthetic route:
[0032]
[0033] The reagents in the above synthetic route are: (a) triphosgene, toluene, reflux, 3 hours; (b) leucine methyl ester hydrochloride, triethylamine, dichloromethane, room temperature, one hour; (c) hydrochloric acid Hydroxylamine, potassium hydroxide, methanol, one hour.
[0034] Specific synthesis method:
[0035] 1) Naphthyl methyl isocyanate (1):
[0036] To the 1,4-dioxane solution of 1-naphthylmethylamine ...
Embodiment 2
[0042] Activity test of target compound to inhibit aminopeptidase N (In vitro)
[0043] 1. Principle: Aminopeptidase N interacts with its substrate (L-leucyl-p-nitroaniline) to produce p-nitroaniline with absorption at 405nm, and the concentration of p-nitroaniline and enzyme activity The size is positively correlated. Determine the content of p-nitroaniline by detecting the absorbance at 405nm, thereby determining the activity of aminopeptidase, which indirectly reflects the degree of inhibition of the enzyme activity by the inhibitor.
[0044] 2. Materials and methods:
[0045] Aminopeptidase N and the substrate L-leucyl-p-nitroaniline were purchased from Sigma
[0046] Solution preparation:
[0047] Buffer solution, prepare pH 7.2, 50mM phosphate buffer solution, and place at room temperature for later use.
[0048] Aminopeptidase N was dissolved in a buffer to make a 0.2mg / mL solution;
[0049] The substrate was dissolved in DMSO to prepare a 0.5 mg / mL solution, and each solution...
Embodiment 3
[0067] In vitro inhibitory activity of target compounds against cell invasion
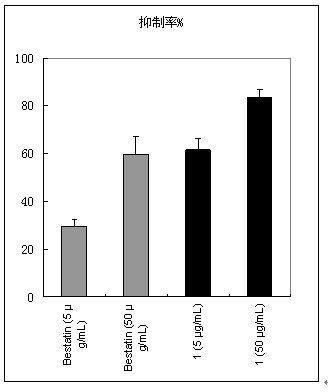
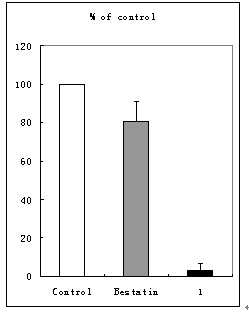
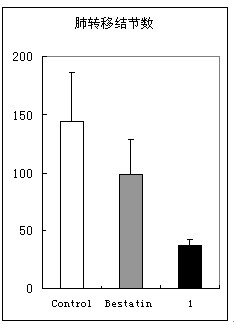
[0068] Compound (I) was tested for the activity of inhibiting tumor cell invasion in vitro, and the results are shown in figure 1 .
[0069] Term description:
[0070] ES-2 is a human ovarian clear cell cancer cell line.
[0071] Bestatin, trade name Ubenmix (Ubenmix), is currently on the market as an immune enhancer for the treatment of leukemia. It is derived from Streptomyces olive ( Streptomyces olivorecticuli ) Small molecule peptide APN inhibitor isolated from the culture medium.
[0072] DMSO: Dimethyl sulfoxide.
[0073] Matrigel: Intercellular matrix extracted from mouse EHS sarcoma
[0074] 1. [Material] ES-2 cell line, pre-coated with Matrigel's Transwell chamber, crystal violet, RPMI-1640 medium containing 1% fetal bovine serum, RPMI-1640 medium containing 10% fetal bovine serum, 24 wells board
[0075] 2. [Method]
[0076] Cell culture The ES-2 cell line was cultured routinely. The logarithm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com