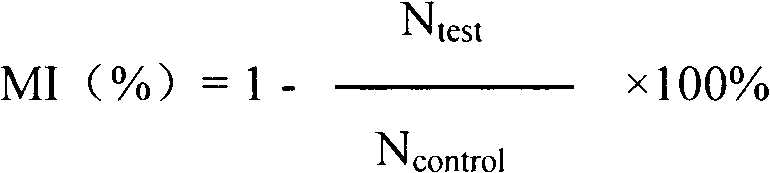Application of angiogenesis inhibitor polypeptide to preparation of medicine for treating tumor and rheumatoid arthritis
An angiogenesis inhibition and vascular inhibition technology, applied in the field of angiogenesis inhibitors and disease treatment, can solve the problems of insufficient dose, irregular medication, persistent and unrelieved symptoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation and Test of Angiogenesis Inhibitor Polypeptide
[0038] The polypeptide is synthesized by solid-phase synthesis, purified by high-performance liquid chromatography, and the molecular weight of the polypeptide is determined by MS, and the purity of the polypeptide is determined by RP-HPLC.
[0039] Polypeptide I, Peptide II, Peptide III and Peptide IV solid-phase synthesis methods use Fmoc-Rink-resin as the starting material, and then use protected amino acids to inoculate each amino acid in turn. After the peptide incorporation is completed, it is fully washed, and then the peptide is cut and post-treated. The crude product of the angiogenesis inhibitor was obtained. The crude product was dissolved, purified twice by preparative high performance liquid phase, and finally concentrated and freeze-dried to obtain the pure product. The method not only ensures the synthesis efficiency, but also improves the purity.
[0040] 1. The steps of receiving peptide are...
Embodiment 2
[0058] Inhibition of several target enzymes by angiogenesis inhibitors.
[0059] Experimental method: Recombinant human matrix metalloproteinase-9 was expressed by Sf9 insect cells. The enzyme was activated with 0.01 μM matrix metalloproteinase-3 active site, and the buffer used was 100 mM Tris / HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl 2 and 0.01% Tween-20. The concentration of matrix metalloproteinase-9 during activation was 92ng / μl (1μM). Enzyme activity is detected by cleaving the fluorescently generated peptide substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH 2 And detect the generated fluorescence value (excitation wavelength = 328nm, detection wavelength = 392nm). All the detection is carried out in 100μl reaction system at 37°C.
[0060] Recombinant human matrix metalloproteinase-8 is detected similarly to -9 and uses the same fluorogenic substrate. The reaction was also carried out in a 100 μl reaction system at 37°C. Add 20 μl of recombinant human matrix metalloproteinas...
Embodiment 3
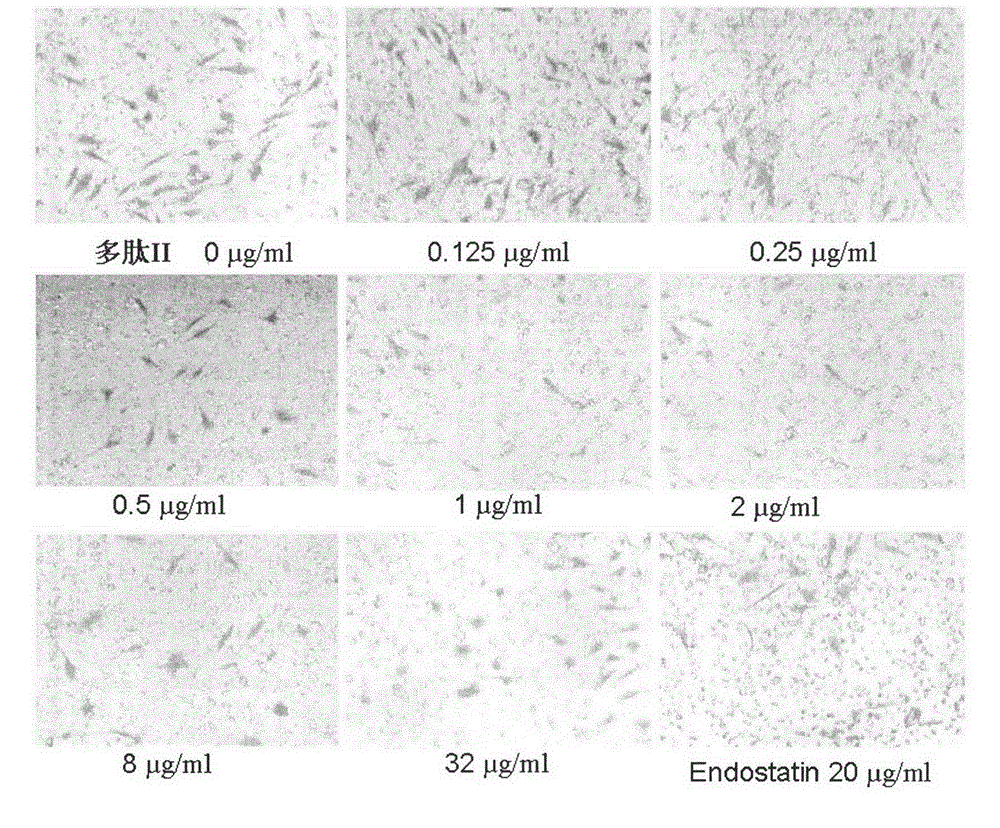
[0067] Migration Inhibition Test of Human Umbilical Vein Endothelial Cells (HUVEC) by Angiogenesis Inhibitor Peptides
[0068] 10 mg / ml Matrigel (BD Company, USA) was diluted 1:2 with HUVEC-specific medium, spread on the transwell membrane, and air-dried at room temperature. The HUVEC cells cultured to the logarithmic growth phase were digested, collected, counted, and the cell concentration was adjusted to 1×10 5 pieces / ml. The cells were inoculated into the transwell chamber, 100 μl per well, and each group of test liquid was added into the chamber. Add 0.6ml of endothelial cell culture medium containing 5% fetal bovine serum and 1% ECGS to the 24-well plate to stimulate cell migration, in 5% CO 2 , and cultivated at 37°C for 24h. The culture medium in the well was discarded, the cells were fixed, stained, rinsed with clean water, observed under a microscope and selected four fields of view to take pictures and count. Migration inhibition (MI) was calculated according to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com