Anti-phosphatidylinositol proteoglycan 3 complete humanized antibody
A technology of phosphatidylinositol and proteoglycan, which is applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc., and can solve problems such as the patent application of Glypican 3 antibody that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1, Synthesis of Glypican 3 (glypican-3) C-terminal polypeptide
[0035] Based on the analysis of the C-terminal structural characteristics of glypican-3, Shanghai Taopu Biotechnology Co., Ltd. was entrusted to artificially synthesize 40 amino acid peptides "AELAYDLDVDDAPGNSQQATPKDNEISTFHNLGNVHSPLK" (524A-563K) 5mg with a purity greater than 95% , for the screening of fully humanized antibodies.
Embodiment 2
[0036] Example 2. Screening of anti-glypican-3 antibody from a fully human large-capacity phage antibody library
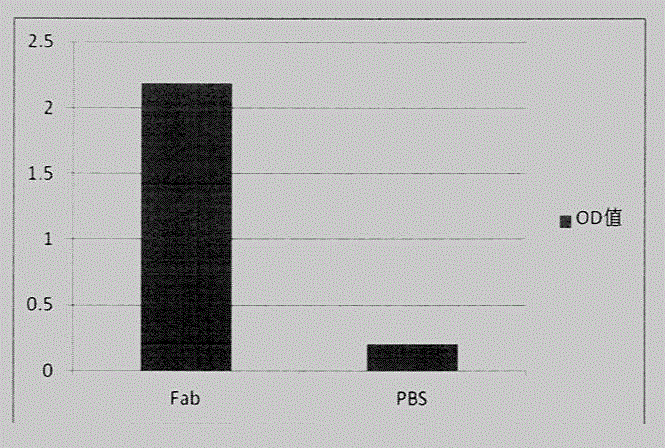
[0037] The large-capacity phage antibody library used for antibody screening has a capacity of 4×10 8 Fab antibody library. First, the phage antibody library was amplified, and the peptides were enriched by coating at 10ug / well. After 6 rounds of elutriation, the enriched phage infected bacteria, and about 1,000 single clones were picked. After a small amount of induction, the supernatant was detected by ELISA, and positive clones were picked, and about 100 positive clones were obtained. The heavy and light chains of these positive clones were re-amplified to establish a peptide-positive phage antibody library, and the peptides were re-enriched for 2 rounds. The enriched phages were used to infect bacteria, plated, and about 200 single clones were picked, and a small amount of induced supernatant was tested by ELISA. The positive clones were picked for sequencin...
Embodiment 3
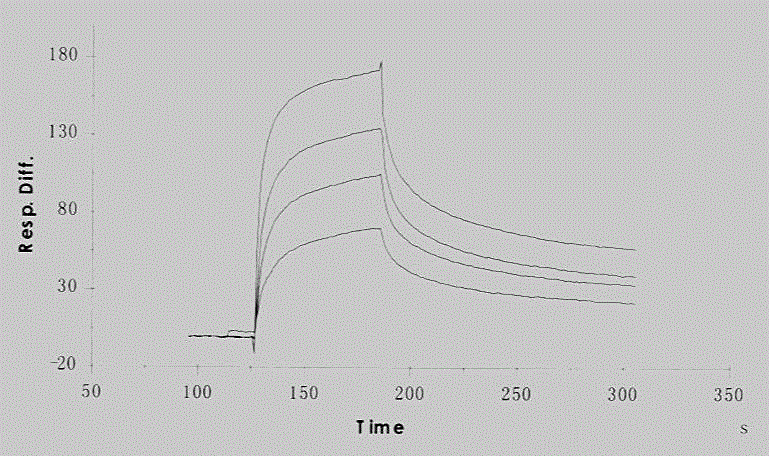
[0052] Example 3, Biacore3000 detects the affinity of Fab antibody and synthetic peptide
[0053] 1. Fixation: Dilute the synthetic peptide to a concentration of 70 μg / ml. Amino coupling method was used to covalently immobilize on carboxymethyl dextran-coated CM5 chip (General Electric product) via primary amine, immobilization buffer 10mM sodium acetate (ph5.0), immobilization amount: 180RU.
[0054] 2. Kinetic analysis:
[0055] PBST (PBS, 0.005% Tween 20) is Runningbuffer; use KineticAnalysisWizard mode; Fab dilution is 37.5, 75, 150, 300, 600, 1200nM concentration gradient; injection time: 1min, dissociation time: 2min, flow rate: 40ul / min
[0056] 3. Regeneration conditions:
[0057] Regeneration solution: 8mMNaOH, injection time: 30s, flow rate: 40ul / min, buffer: 40siniection
[0058] 4. Result analysis:
[0059] Fitting software: BIAevaluation4.1software, fitting model: 1:1bindingmodel. ka(1 / Ms)=1.03×10 5 , kd(1 / s)=6.73×10 -3 , KD(M)=6.54×10 -8 , anti-glypican...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com