Butyl phthalide medicinal composite, preparation method and sustained-release preparation thereof
A technology for drug complexes and sustained-release preparations, applied in drug combinations, drug formulations, drug delivery, etc., can solve the problems of cumbersome and complex preparation processes, poor drug stability, and complex components, and achieve good economy and high drug stability , The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
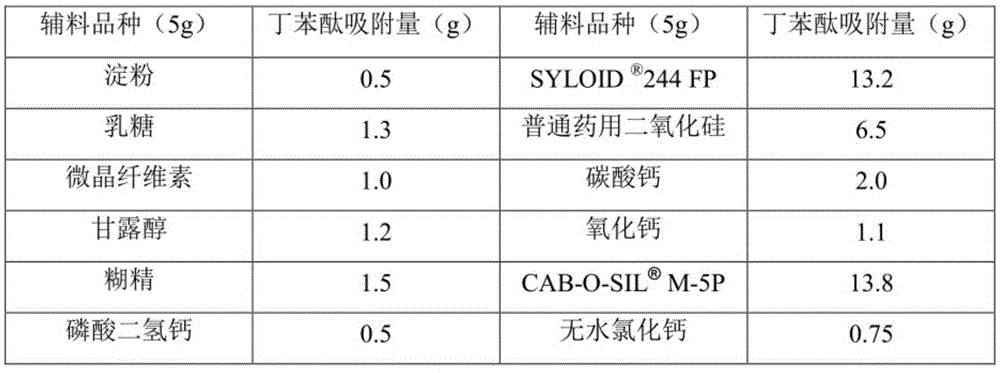
[0029] Weigh 500 g of ordinary medicinal silicon dioxide and 100 g of racemic butylphthalide, and spray the butylphthalide into the silicon dioxide under stirring and mix evenly to obtain a solid powdery butylphthalide drug complex. The prepared powder can be prepared into various dosage forms such as granules, capsules or tablets by adding suitable auxiliary materials.
Embodiment 2
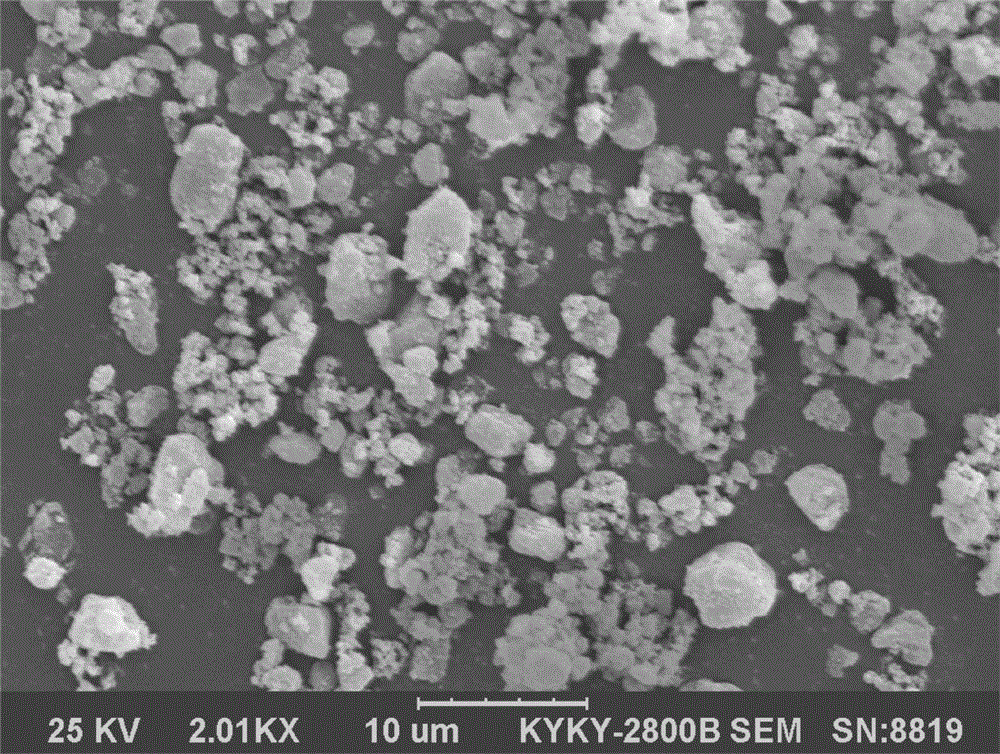
[0031] Weigh silica M-5P500g and racemic butylphthalide 500g, put the silicon dioxide in the fluidized bed, start the machine to make the silicon dioxide in a fluidized boiling state, spray butylphthalide into it, and get solid powdery butylphthalide drug compound The SEM image of the prepared butylphthalide drug complex is as follows figure 1 shown. The prepared powder can be prepared into various dosage forms such as granules, capsules or tablets by adding suitable auxiliary materials.
Embodiment 3
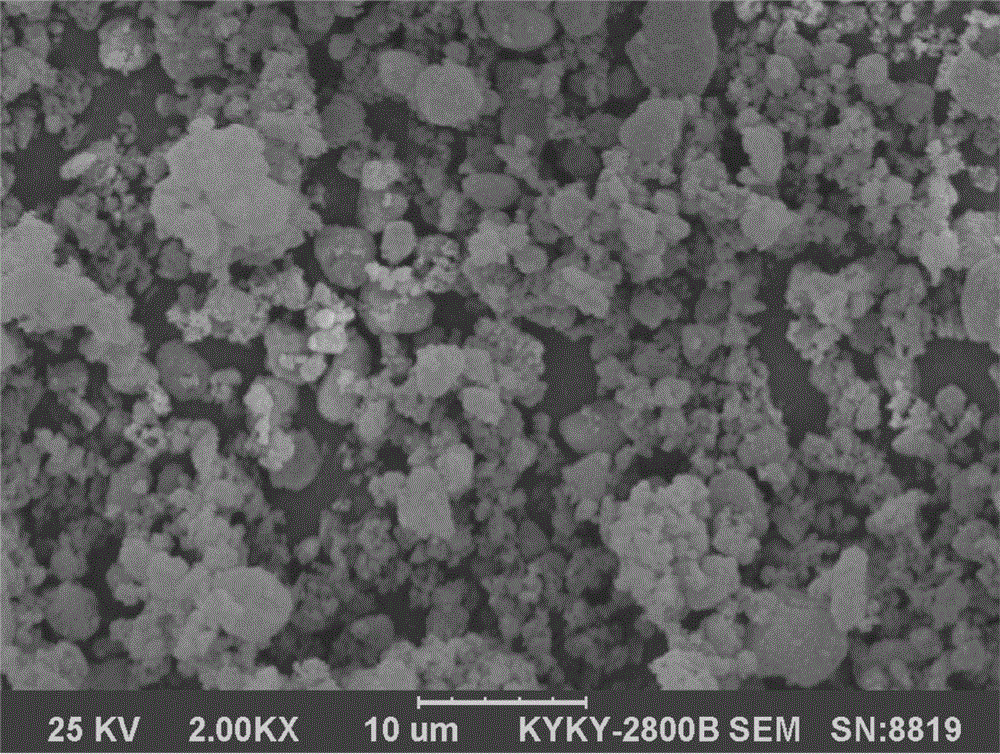
[0033] Weigh silica 244FP500g and racemic butylphthalide 1000g, put the silicon dioxide in the granulator, stir continuously and at the same time spray the butylphthalide into the silicon dioxide and mix evenly to get the solid powdery butylphthalide drug complex. The SEM figure of the prepared butylphthalide drug complex is as follows figure 2 shown. The prepared powder can be prepared into various dosage forms such as granules, capsules or tablets by adding suitable auxiliary materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com