Synthesized micromolecule compound capable of conveying bioactivator and application thereof
A technology for small molecular compounds and biologically active substances, applied in the field of small molecular compounds, can solve the problems of inability to meet the oral delivery of hydrophilic macromolecular drugs, poor stability, inability to achieve, etc., achieve convenient oral administration, improve stability, The effect of improving the absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
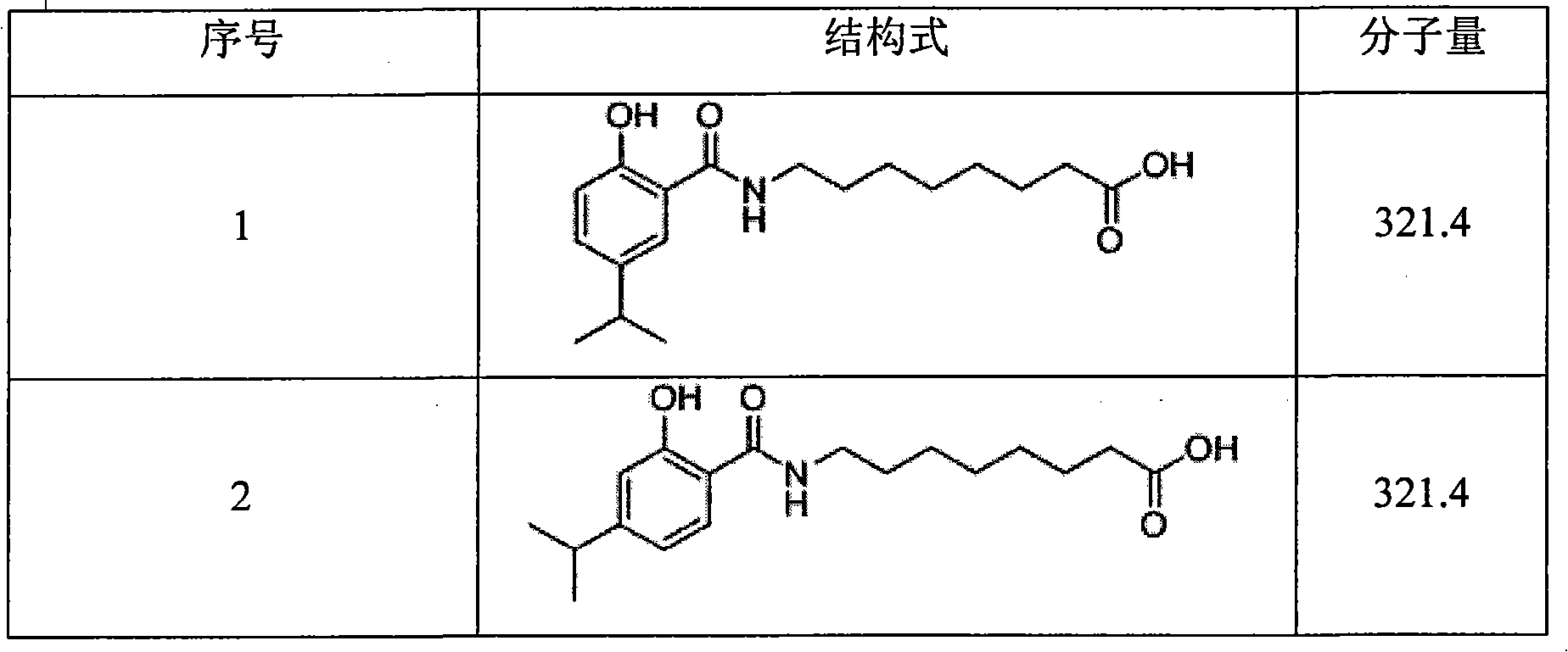
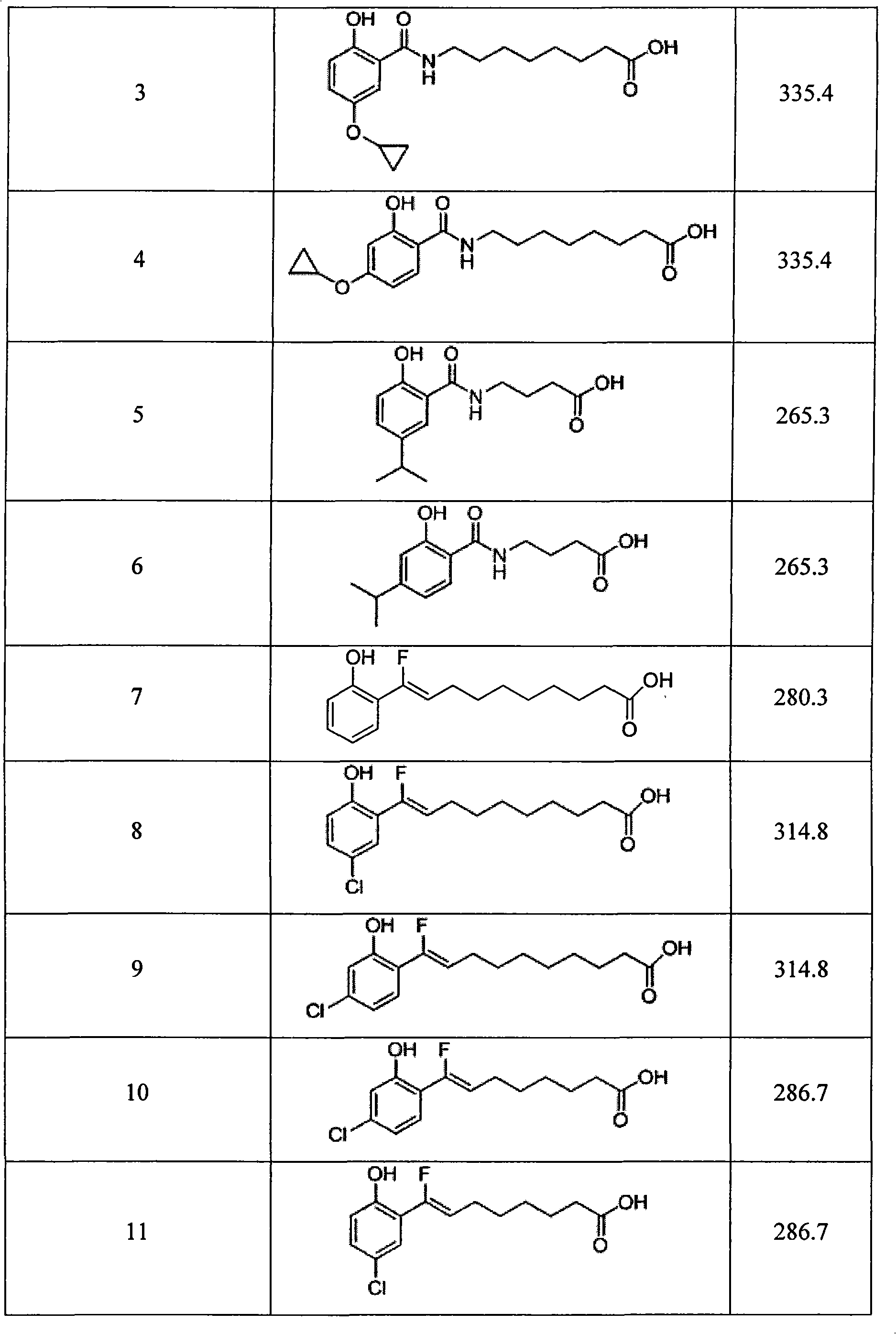
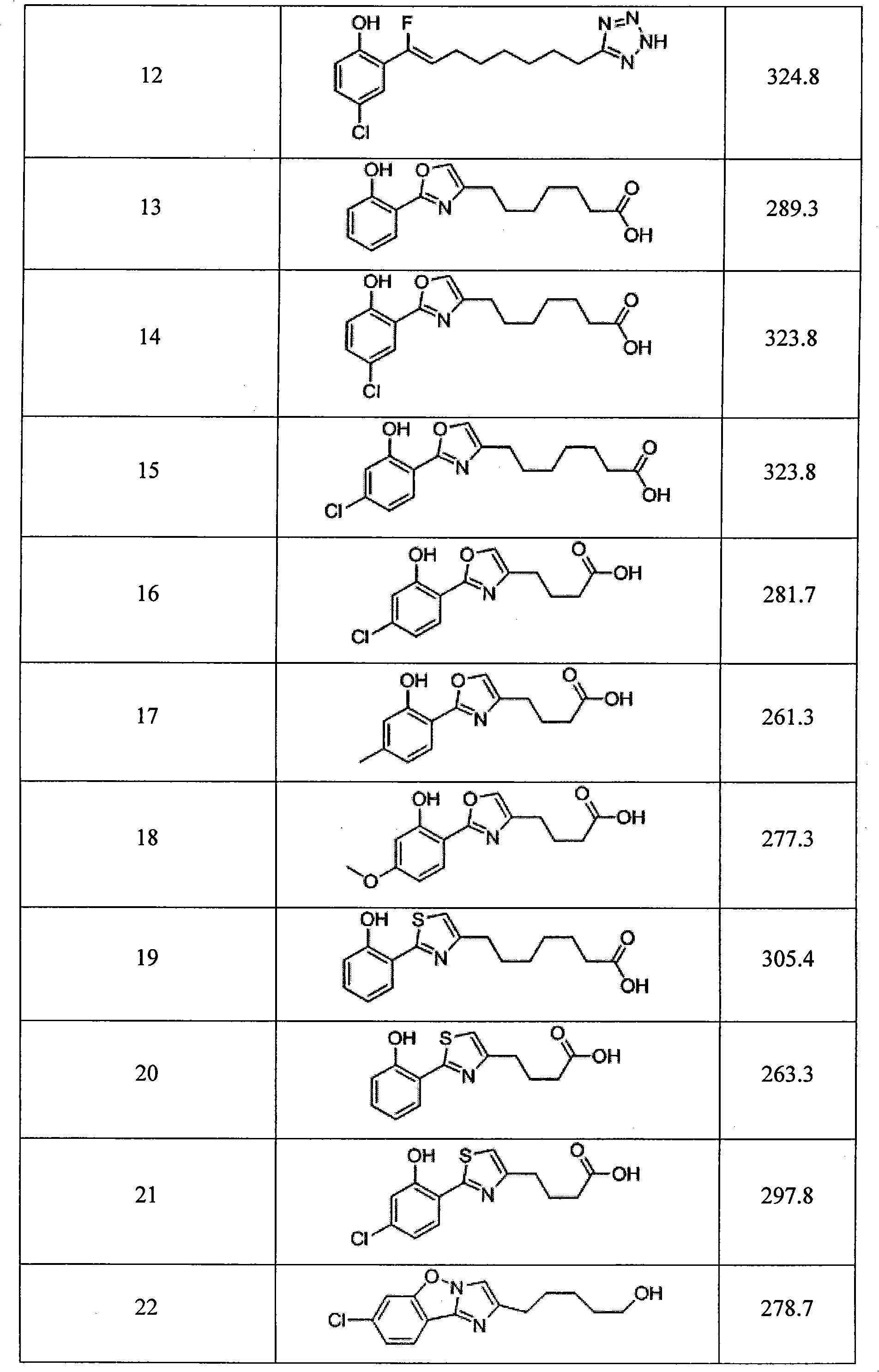
Image
Examples
Embodiment 1
[0040]
[0041] Preparation of compound 5: add 1-(3-dimethylaminopropyl)-3-ethyl to a solution of 2-hydroxy-5-isopropylbenzoic acid (1.0 equivalent) in dichloromethane (30 ml) Carbodiimide hydrochloride (1.0 equivalent) and 4-dimethylaminopyridine (2 equivalent). After stirring this solution at 0°C for 20 minutes, add methyl 4-aminobutyrate (1.0 equivalent) to the solution. ) In dichloromethane (10 mL). After the addition, the reaction mixture was gradually warmed to room temperature and stirred at room temperature for 12 hours.
[0042] The solution was transferred to a separatory funnel, and washed with 1 equivalent hydrochloric acid solution (2×20 mL), water (2×20 mL), saturated sodium bicarbonate solution (2×20 mL), and saturated brine (40 mL). It was dried with anhydrous magnesium sulfate, filtered, and concentrated in vacuo. Subsequently, the residue was dissolved in tetrahydrofuran (30 mL) and sodium hydroxide solution (2 equivalents) was added. The mixture was stirred at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com