Metal organic framework nanoparticles for oral protein administration and preparation method of metal organic framework nanoparticles
A metal-organic framework and nanoparticle technology, applied in the field of pharmacy, can solve the problems of low protein oral bioavailability and complex product preparation process, and achieve good biocompatibility, slow and controlled release kinetics, low price, and convenient protein The effect of oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
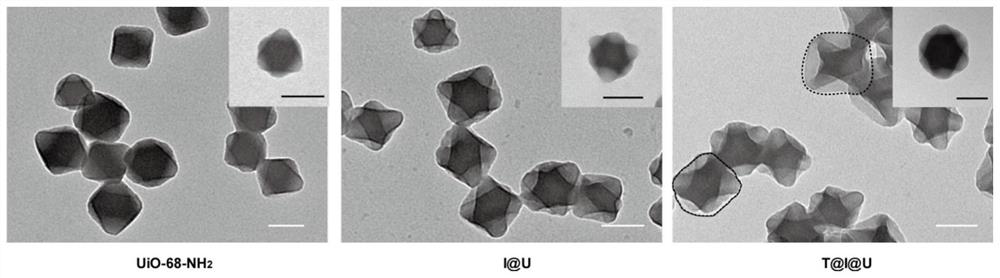
[0029] Example 1 Preparation and Characterization of Oral Insulin Metal Organic Framework Nanoparticles (T@I@U)
[0030] Preparation of UiO-68-NH by Solvothermal Method 2 : amino-TPDC (10.5mg, 0.031mmol) and ZrOCl 2 ·8H 2 O (8.3 mg, 0.026 mmol) was dissolved in 2 mL DMF. The mixture was sonicated for 5 minutes until it was clear. 40 µL of acetic acid was added to the mixture, which was then heated at 90 °C for 6 h. The mixture was centrifuged at 11000 rpm and washed 3 times with DMF to obtain purified UiO-68-NH 2 . Continue to centrifuge 3 times at 11000rpm to exchange DMF into ddH 2 O.
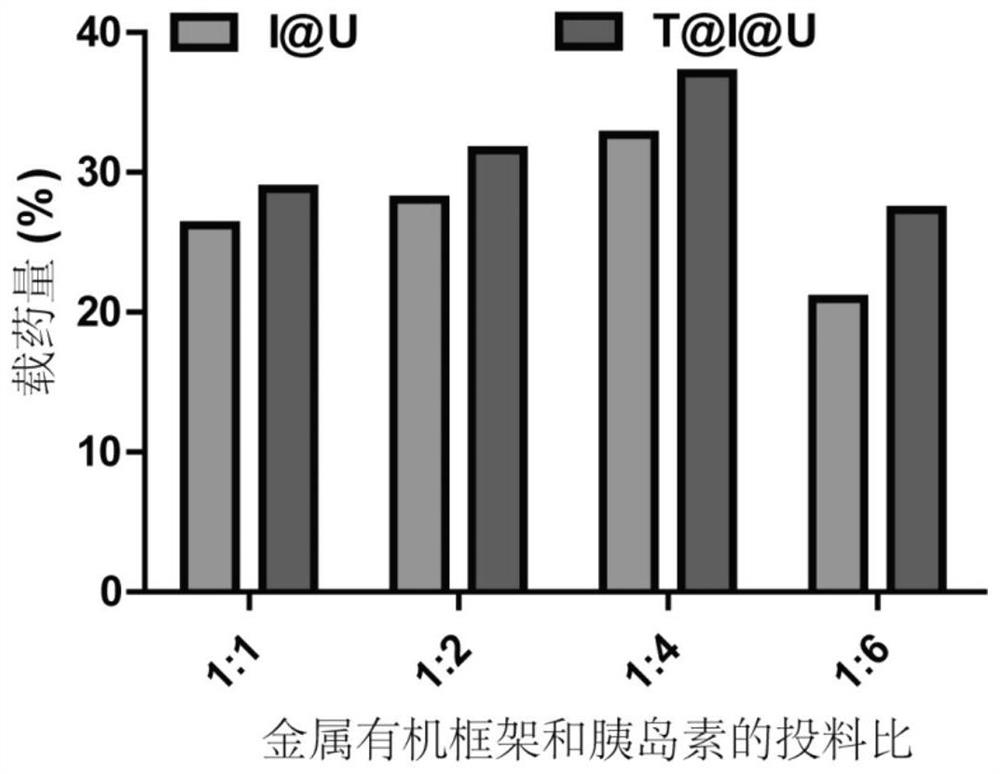
[0031] Under the condition of stirring at room temperature, 50-300μL of insulin solution (20mg / mL, pH=2.0, dilute hydrochloric acid as solvent) was added dropwise to 4mL of newly synthesized UiO-68-NH 2 (0.25mg / mL) aqueous solution, stirring continuously for 12 hours to obtain insulin@MOF nanoparticles (I@U). Subsequently, 40 μL of transferrin aqueous solution (5 mg / mL) was added t...
Embodiment 2
[0033] Example 2 Detection of Insulin Activity
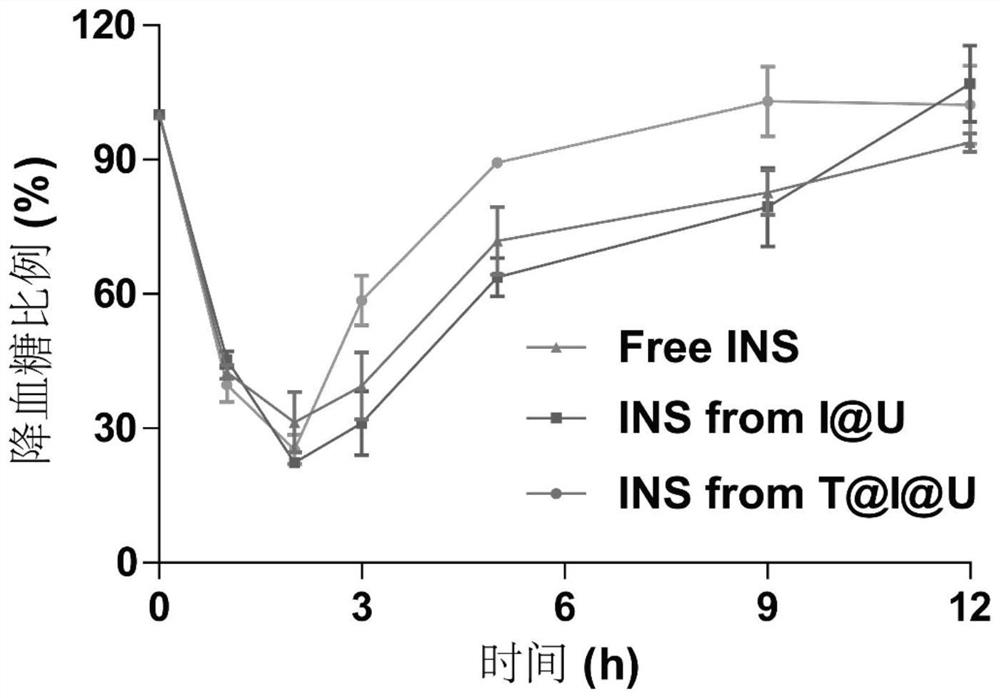
[0034] In order to verify that insulin still has considerable biological activity during the preparation of nano-preparations and the release of nano-preparations, in this example, I@U and T@I@U freshly prepared according to the method in Example 1 were added at 37 Immerse in 1 mL of PBS buffer for 24 hours at °C, and centrifuge at low temperature (11000 rpm) to obtain the supernatant. The released insulin content in the supernatant was measured using a BCA kit, and then the released insulin (5 IU / kg) and the unloaded insulin (5 IU / kg) were administered to SD rats overnight fasted by subcutaneous injection. The blood glucose concentration of rats at different time points was measured. The result is as image 3 As shown, it shows that the insulin metal organic framework nanoparticles prepared by the present invention will not damage the biological activity of insulin during the preparation process.
Embodiment 3
[0035] Example 3 Research on the protective properties of T@I@U on insulin
[0036] To determine UiO-68-NH 2 Whether insulin can be protected from digestive enzymes, freshly prepared I@U and T@I@U according to the method in Example 1 were incubated in HBSS buffer containing trypsin (1 mg / mL) at 37°C. Aliquots (100 μL) were withdrawn at specific time intervals, 200 μL of DMSO containing 0.1% trifluoroacetic acid was added to stop the enzymatic reaction, and the concentration of insulin in the solution was determined with an Elisa kit. Such as Figure 4 As shown, I@U and T@I@U can well protect insulin from destruction within 2 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com