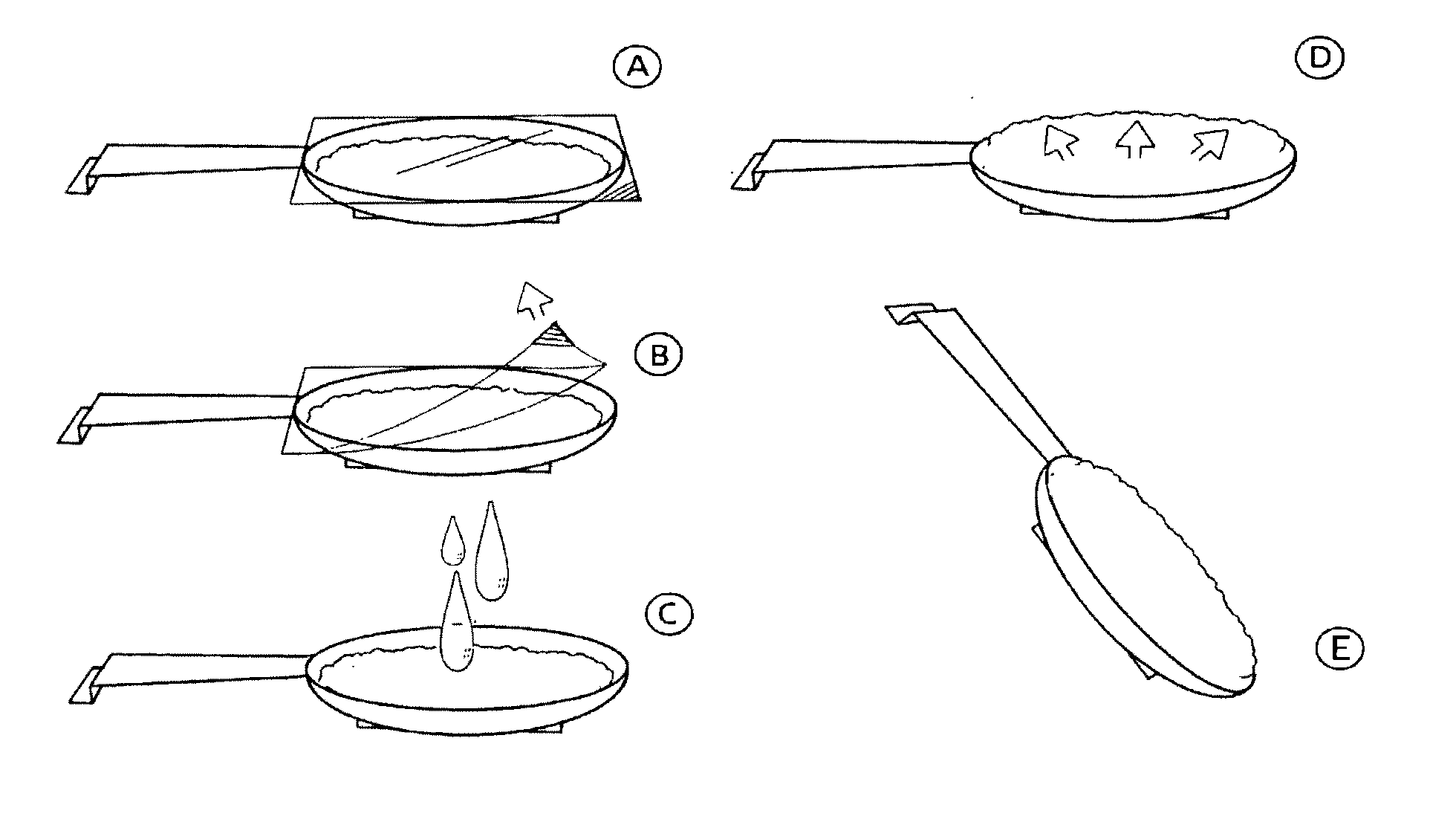
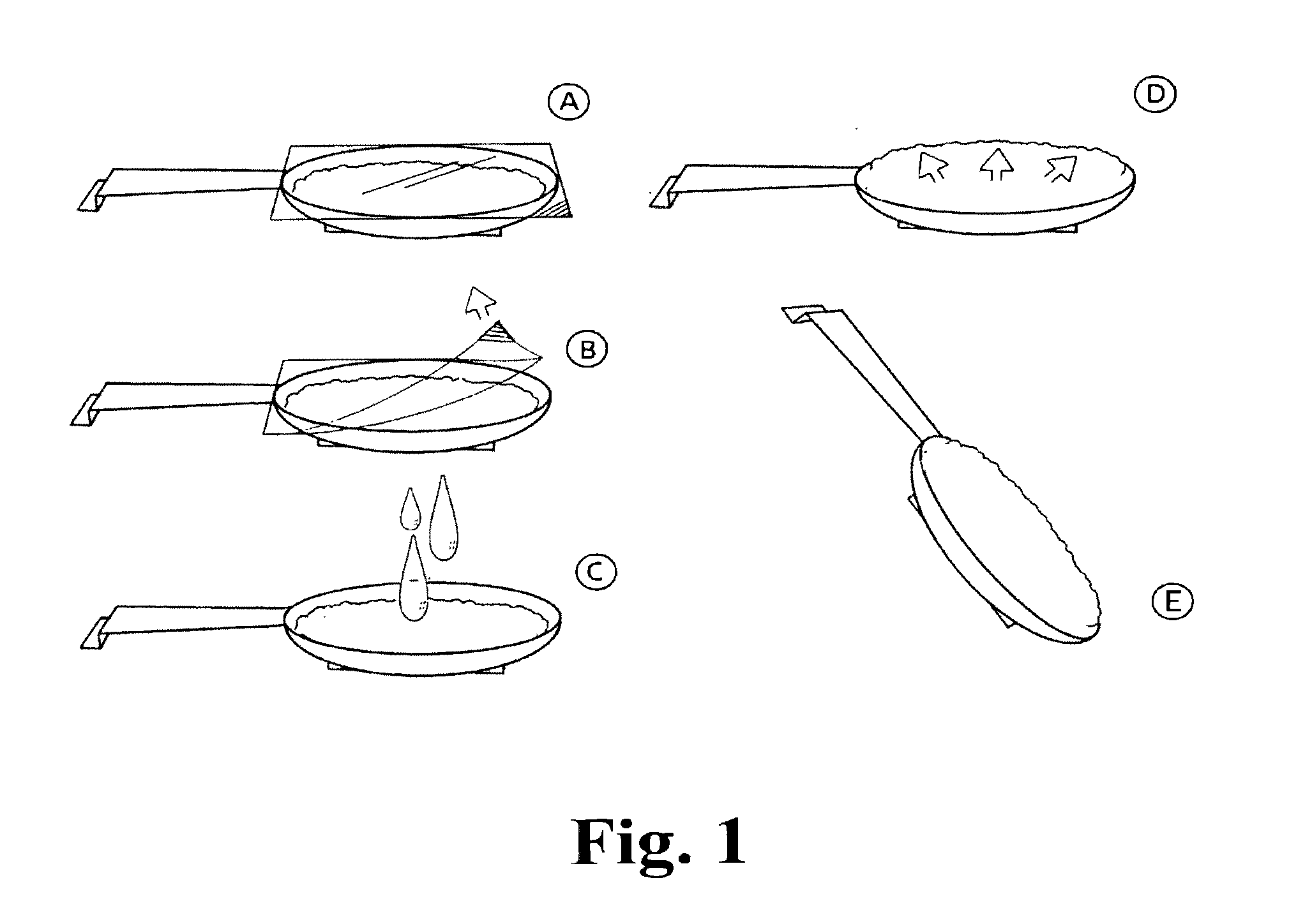
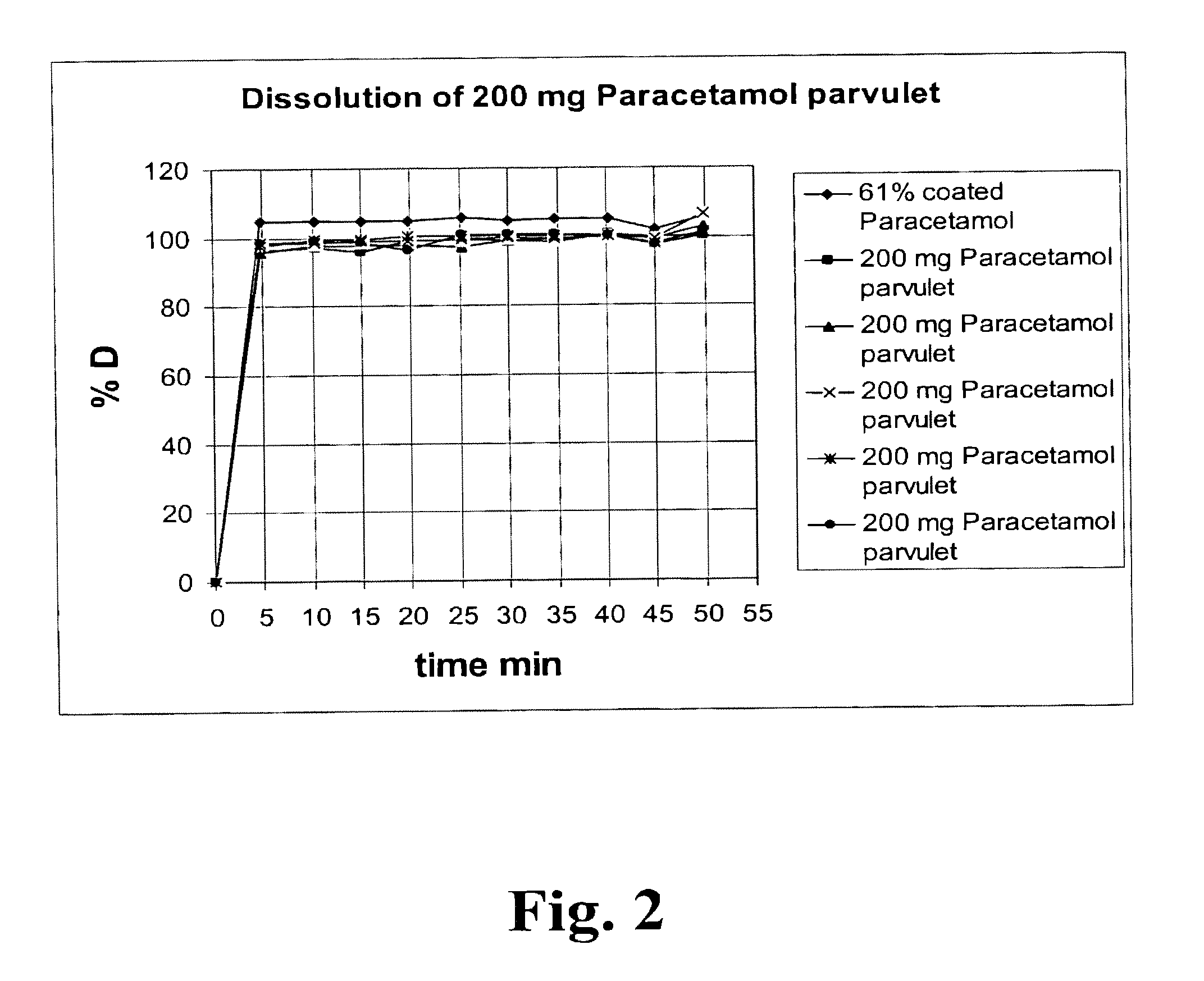
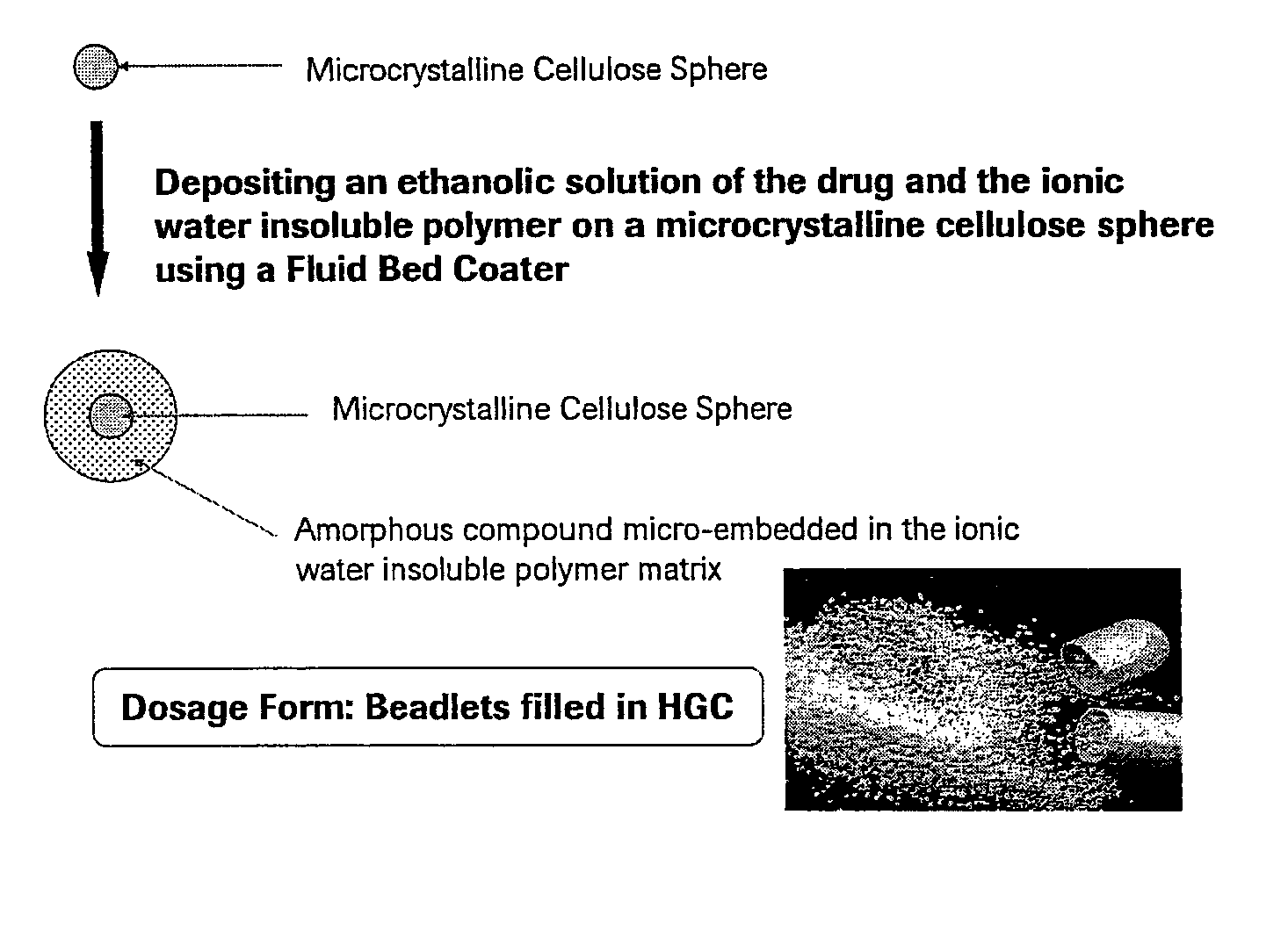
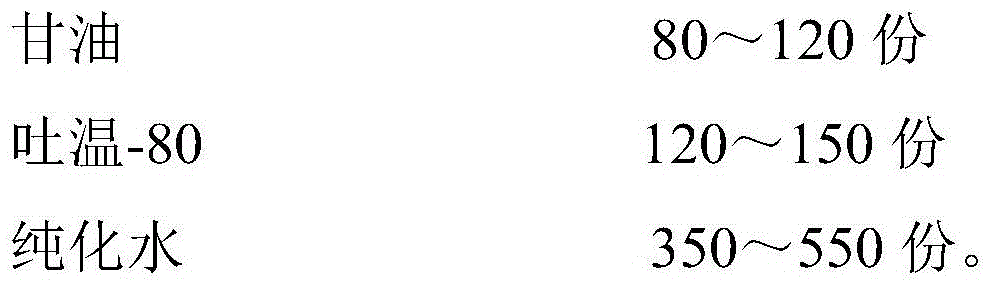Patents
Literature
57 results about "Gel Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
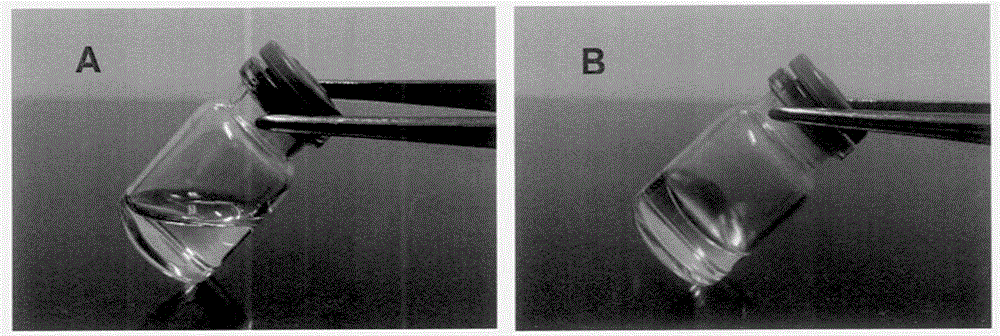
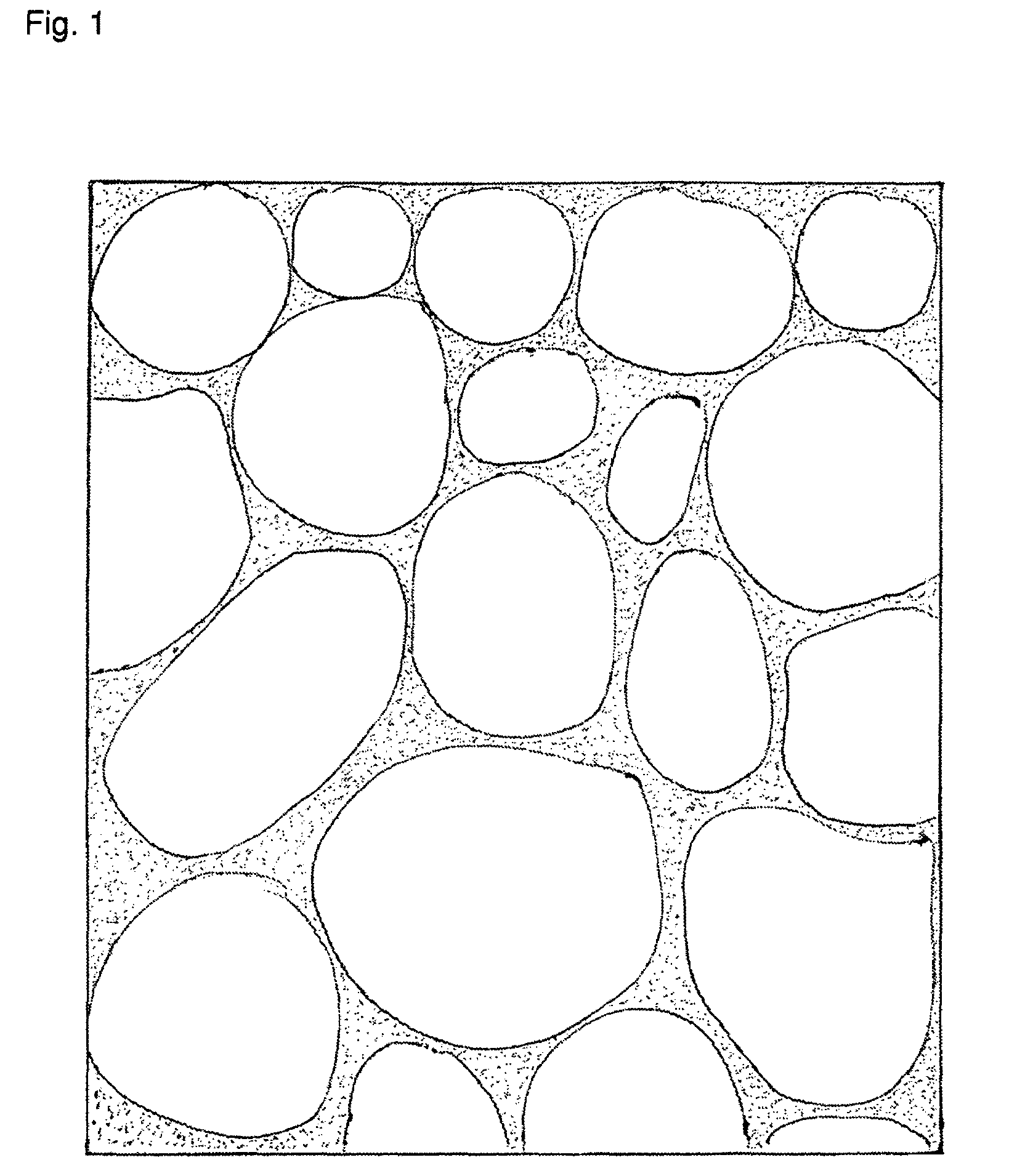
A semisolid (1) dosage form that contains a gelling agent to provide stiffness to a solution or a colloidal dispersion (2). A gel may contain suspended particles. Note 1: A semisolid is not pourable; it does not flow or conform to its container at room temperature. It does not flow at low shear stress and generally exhibits plastic flow behavior. Note 2: A colloidal dispersion is a system in which particles of colloidal dimension (i.e., typically between 1 nm and 1 micrometer) are distributed uniformly throughout a liquid.
Simethicone solid oral dosage form
The present invention provides a composition for forming a compressed solid dosage form that is a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22. Also included are solid dosage forms made from a free-flowing compressible admixture of simethicone, an adsorbant, and an optional active agent, wherein the weight ratio of simethicone to adsorbent is at least 1:2.22.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Modified release dosage forms
In one embodiment a dosage form comprises at least one active ingredient and a molded matrix which comprises 10-100% of a material having a melting point of less than about 100 degrees C. selected from the stamp consisting of thermoplastic polyalkylene oxides, low melting hydrophobic materials, thermoplastic polymers, thermoplastic starches and combinations thereof, and the matrix is capable of providing modified release of the active ingredient upon contacting of the dosage form with a liquid medium. The dosage form may additionally comprise uncoated particles which may contain at least one active ingredient. In another embodiment, a dosage form comprises at least one active ingredient, a plurality of particles and a molded matrix, wherein at least a portion of the particles are coated. The coated particles, the matrix or both may comprise at least one active ingredient, and the coated particles or the matrix or a combination thereof is capable of providing modified release of the active ingredient upon contacting of the dosage form with a liquid medium.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
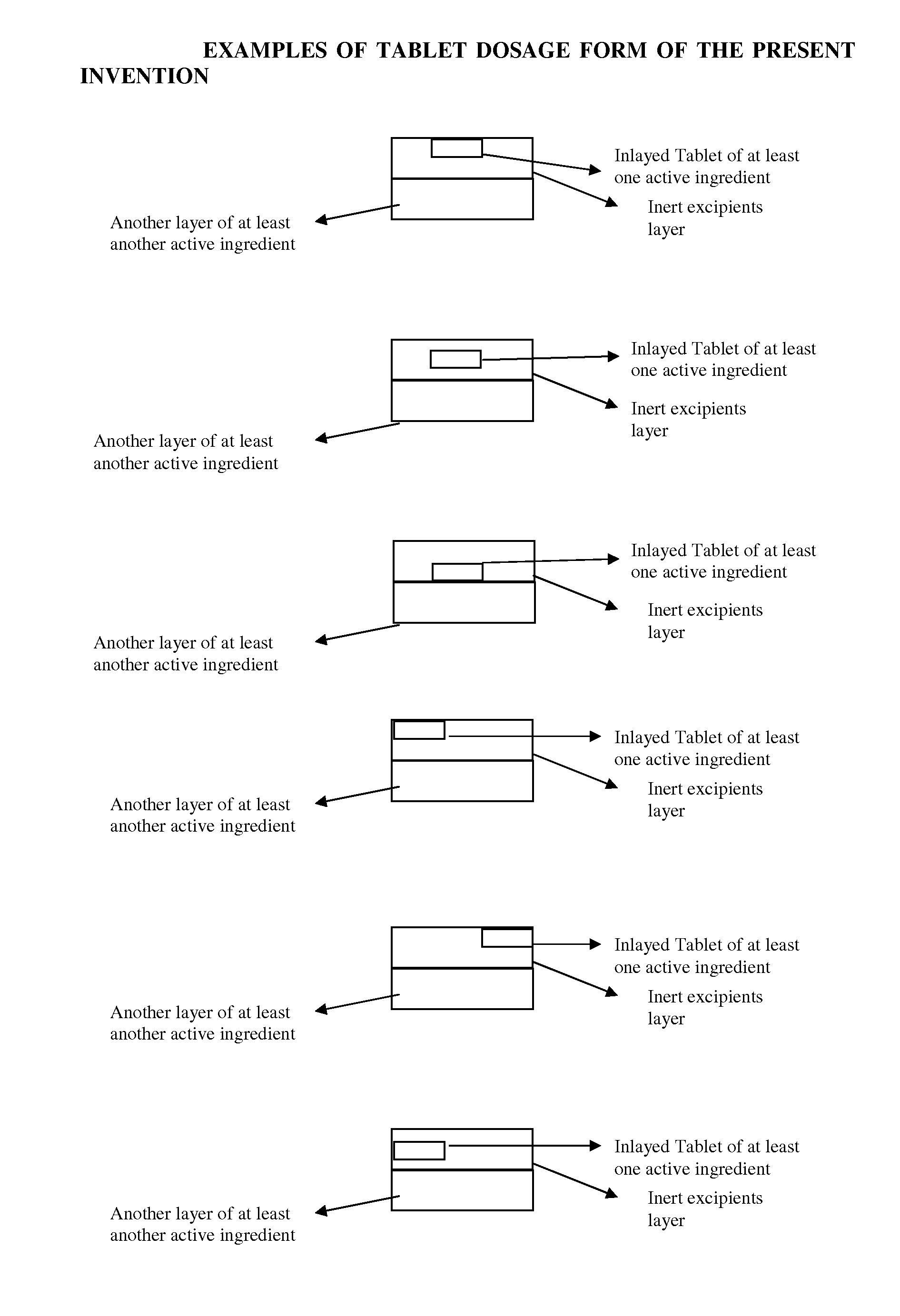
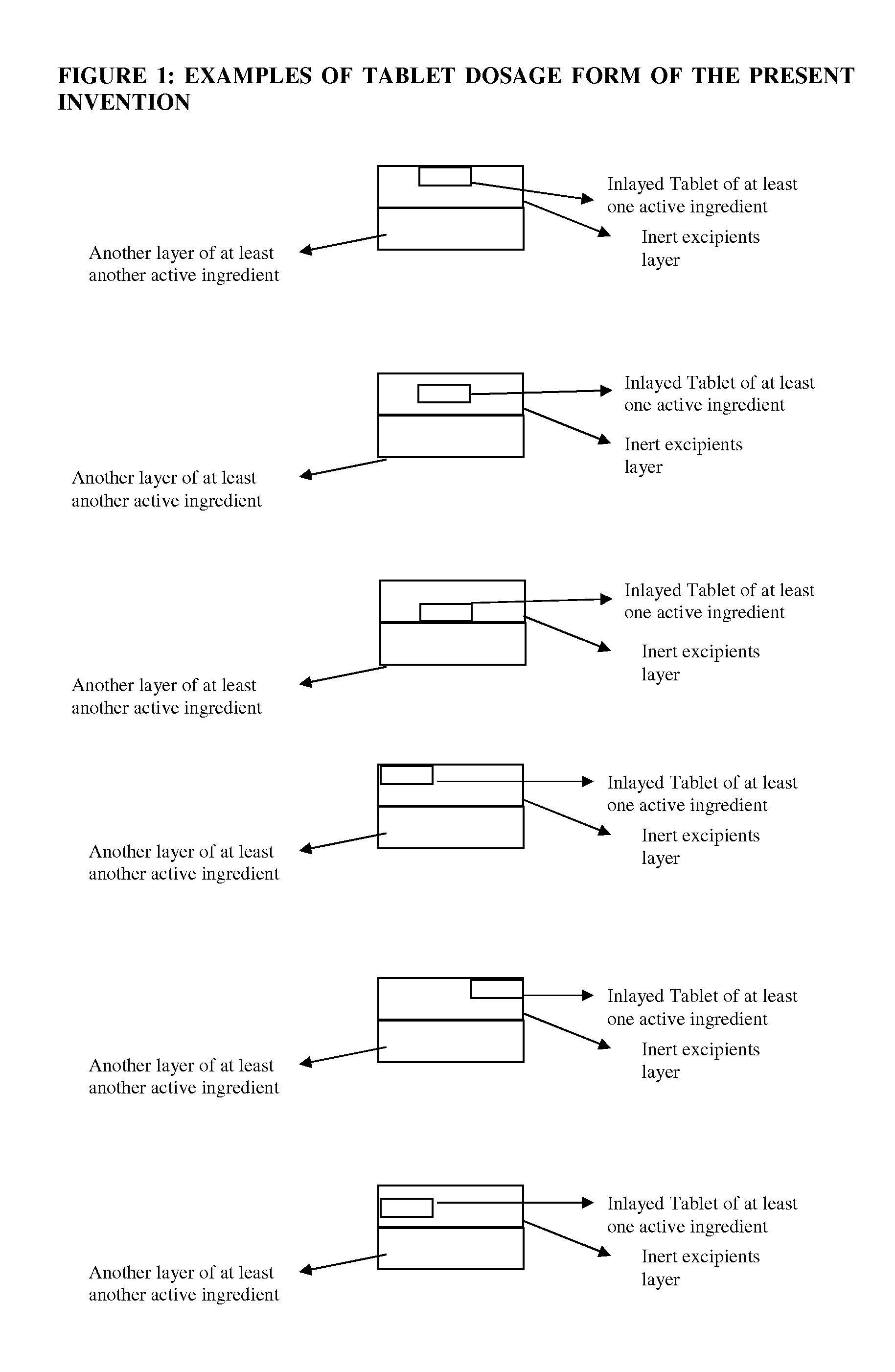
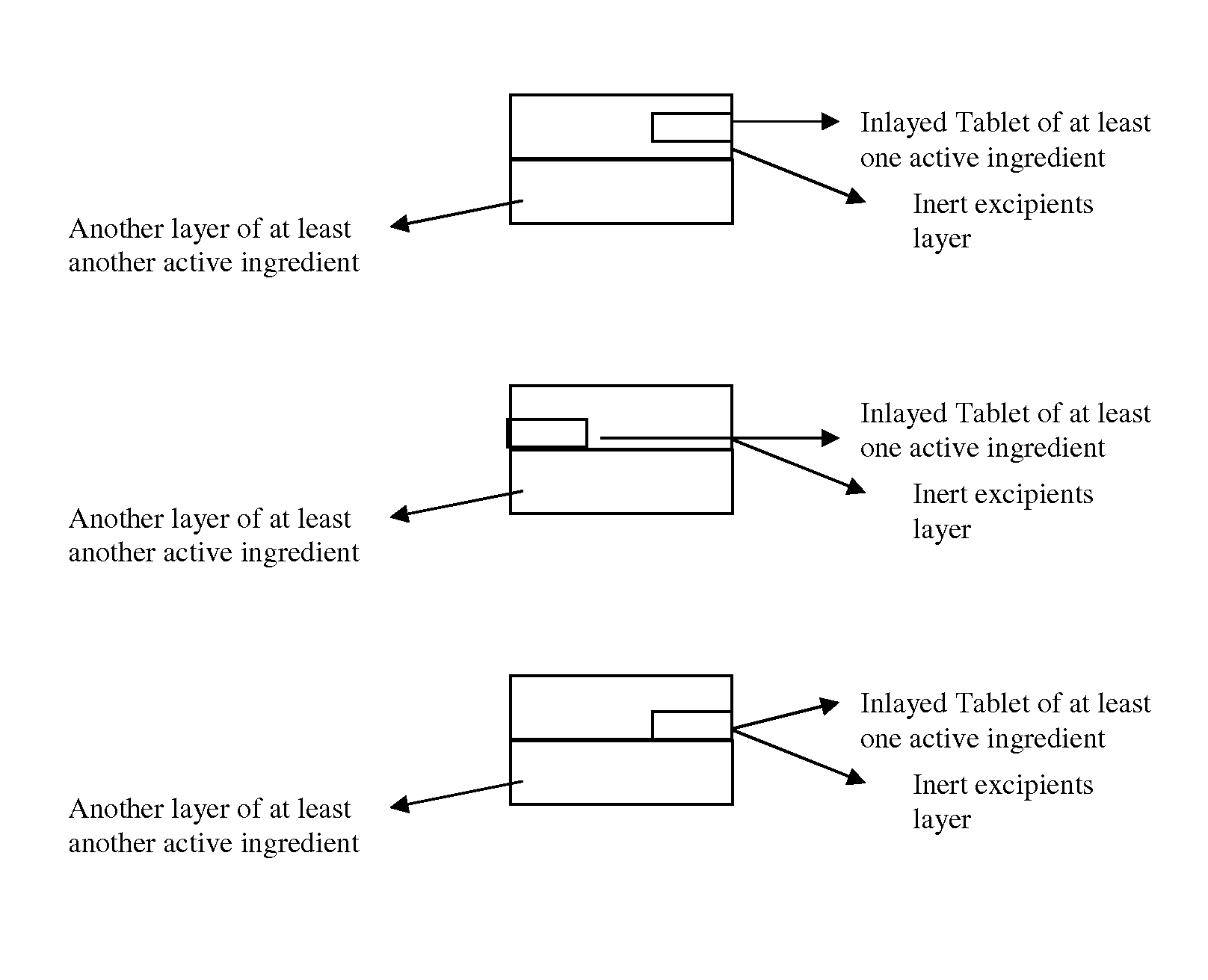
Novel tablet dosage form
The present invention relates to novel tablet dosage forms and methods of preparing these forms, which can be used for different classes of pharmaceutical active ingredients posing stability issues in a single unit system. The dosage form includes a first layer, which includes a tablet of one or more active pharmaceutical ingredients, which is inlayed in the first layer along with other pharmaceutically acceptable excipients, and a second layer that includes one or more active pharmaceutical ingredients optionally with other pharmaceutically acceptable excipients.
Owner:WOCKHARDT LTD
Dosage form comprising an active ingredient and a plurality of solid porous microcarriers
The present application provides a dosage form and related methods for making the dosage form. The dosage form generally comprises a hydrophilic active ingredient, a plurality of solid, porous microcarriers, each having a hydrophobic surface, an optional hydrophobic encapsulant, and a hydrophilic delivery agent, wherein (i) the hydrophilic active ingredient is associated with the plurality of solid, porous microcarriers, (ii) the plurality of solid, porous microcarriers is encapsulated by the hydrophobic encapsulant, and (iii) the hydrophilic delivery agent is physically separated from a majority of the hydrophilic active ingredient by a boundary between the hydrophilic delivery agent and the hydrophobic encapsulant. In some embodiments, the dosage form is for topical application. In some additional embodiments, the plurality of solid, porous microcarriers is formed by modifying the microcarriers to increase their hydrophobicity.
Owner:BIOPHARMX
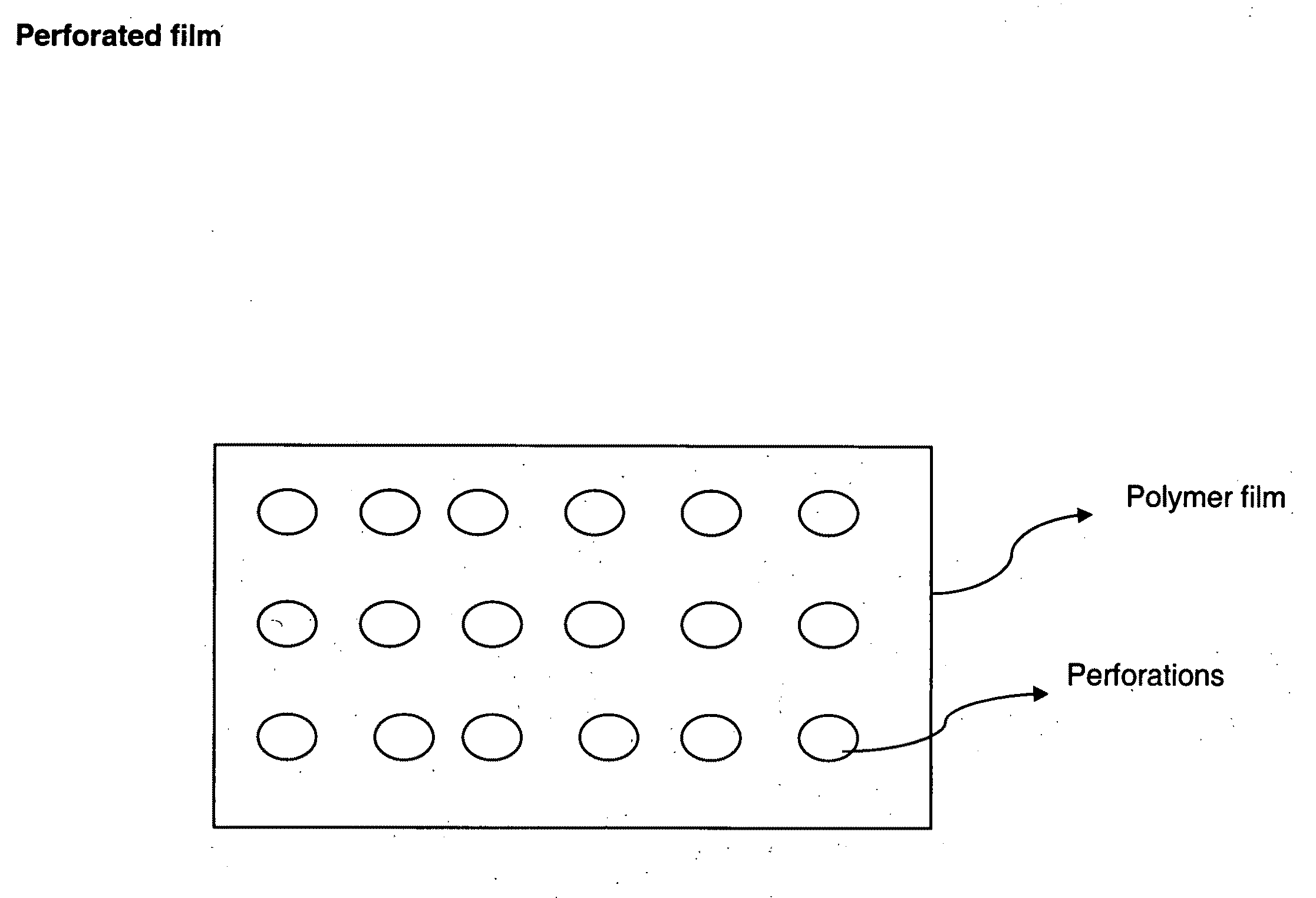
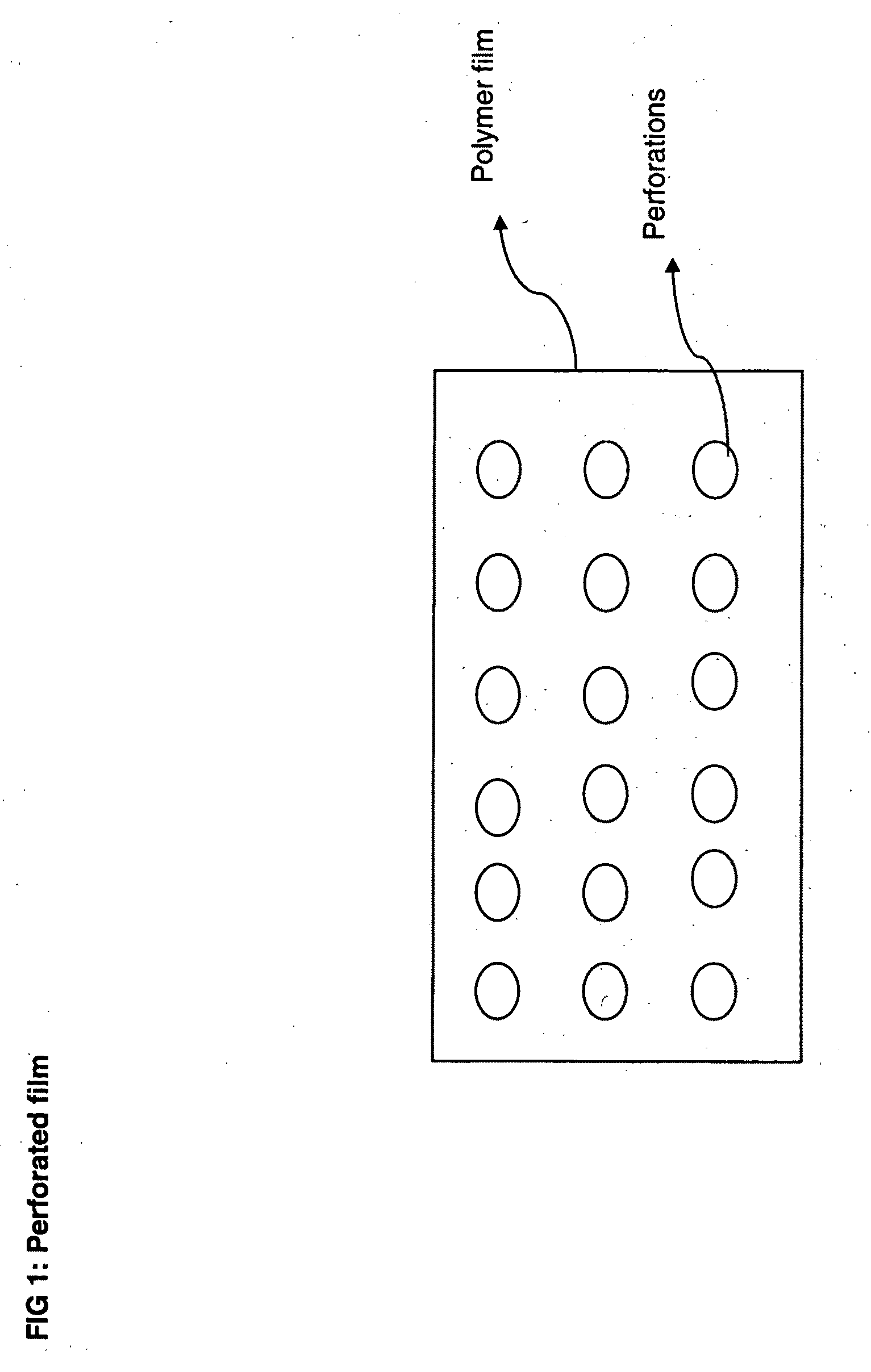
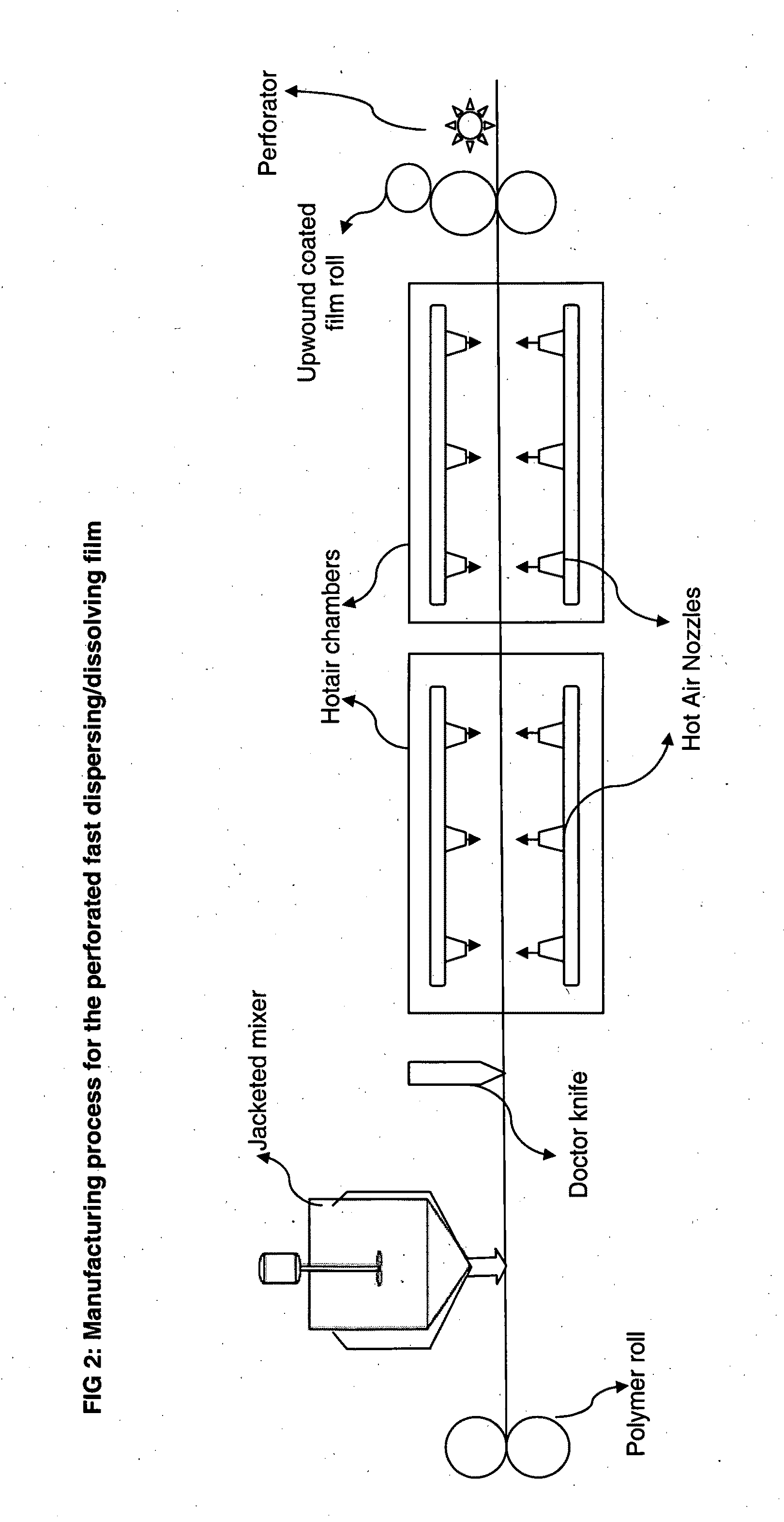
Perforated water soluble polymer based edible films
InactiveUS20090047350A1High drug loadingImprove abilitiesPowder deliveryOrganic active ingredientsSolubilityActive agent
Owner:BANGALORE RAMESH
Methods for oral administration of active drugs
InactiveUS20090155363A1Oral administration is convenientBiocidePowder deliveryCo administrationOral medication
The present invention relates to methods that facilitate the oral administration of active drugs to a patient. Specifically, the methods of the present invention may utilize compositions comprising an active drug and a gelling agent that provides an easily consumable gel dosage form and the active drug is homogenously mixed within the gel.
Owner:MAIBACH TODD
Doxycycline metal complex in a solid dosage form
The present invention is a solid dosage form of a doxycycline metal complex. The present invention also includes a process for making a doxycycline metal complex in a solid dosage form. The process comprises the steps of (i) providing an aqueous solution of doxycycline or a physiologically acceptable salt thereof; (ii) admixing a metal salt with the aqueous solution; (iii) admixing a base to increase the pH of the aqueous solution, thereby forming a suspension of doxycycline metal; and (iv) drying the suspension, thereby forming a dry granulation of doxycycline metal complex.
Owner:APTALIS PHARMA
Solid dosage form of enteric solid dispersion and method for producing the same
Provided are a solid dosage form comprising an enteric solid dispersion that allows a drug in the preparation to be rapidly dissolved without compromising the solubility of the solid dispersion, and a method for producing the same. More specifically, provided is a solid dosage form comprising an enteric solid dispersion comprising a poorly soluble drug, an enteric polymer and a disintegrant, wherein the disintegrant is low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g. Moreover, provided is a method for producing a solid dosage form comprising an enteric solid dispersion, the method comprising steps of: spraying an enteric polymer solution in which a poorly soluble drug has been dispersed or dissolved, on a powder of low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g and serving as a disintegrant; and granulating the resultant; and drying.
Owner:SHIN ETSU CHEM IND CO LTD
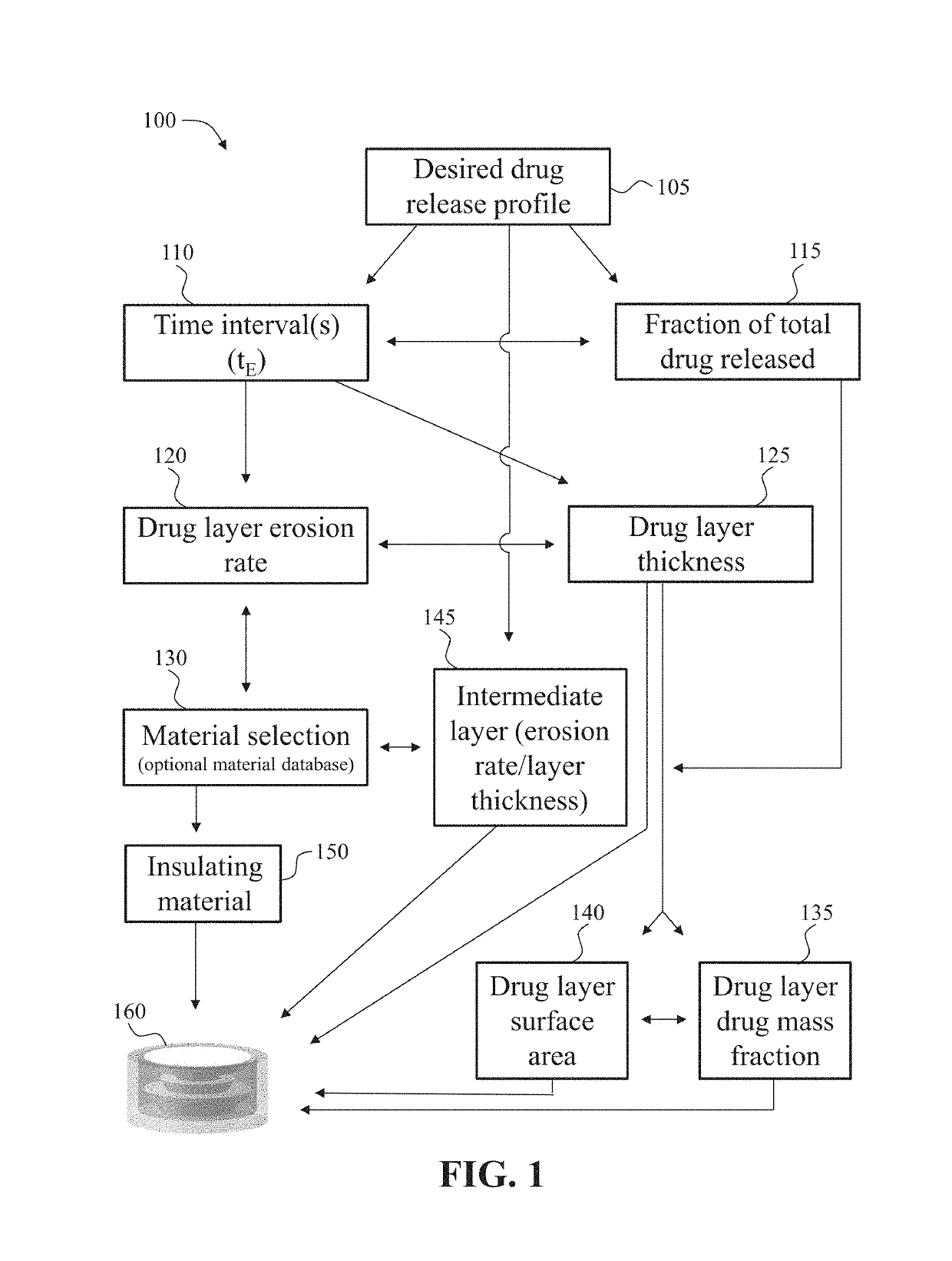
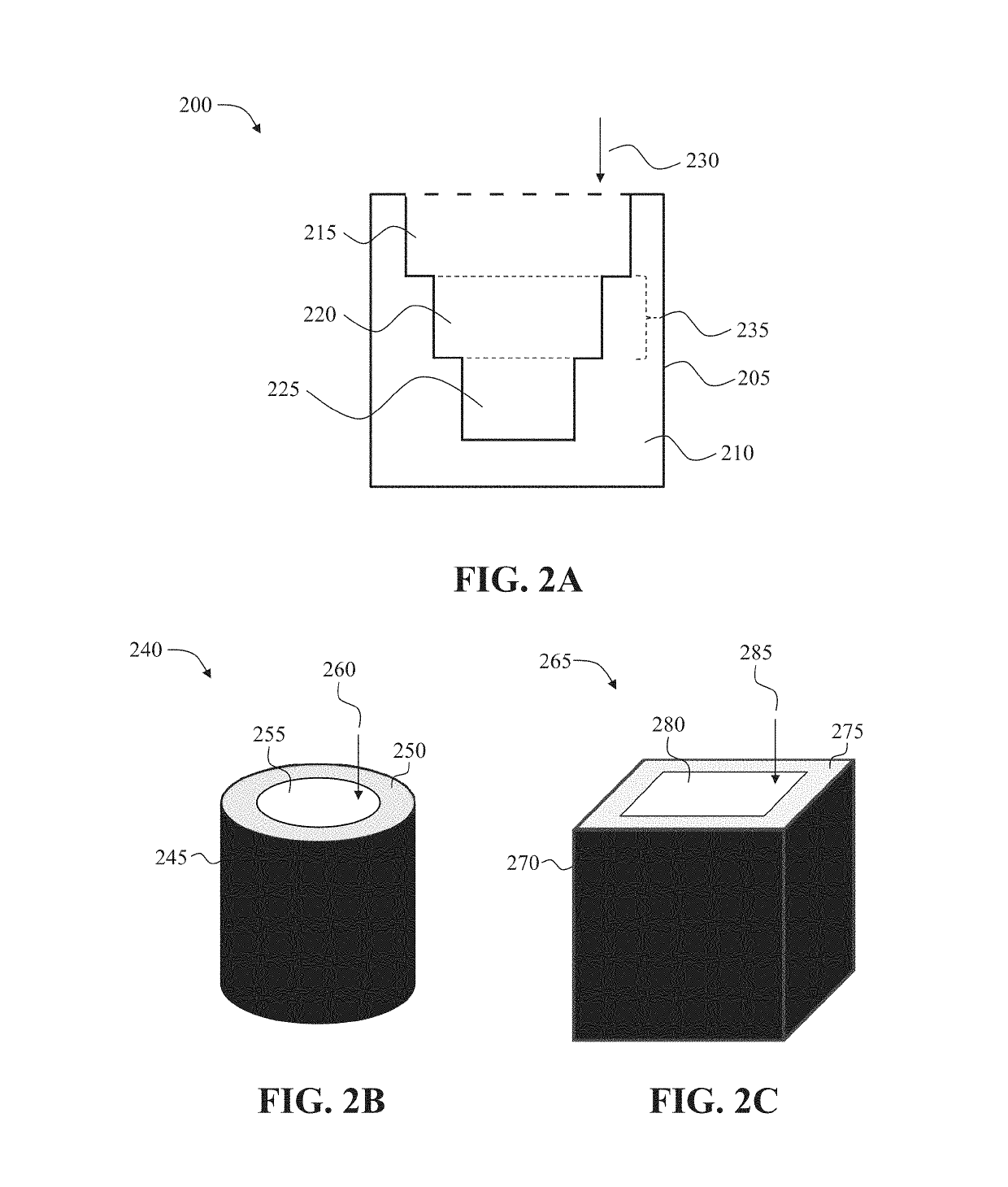
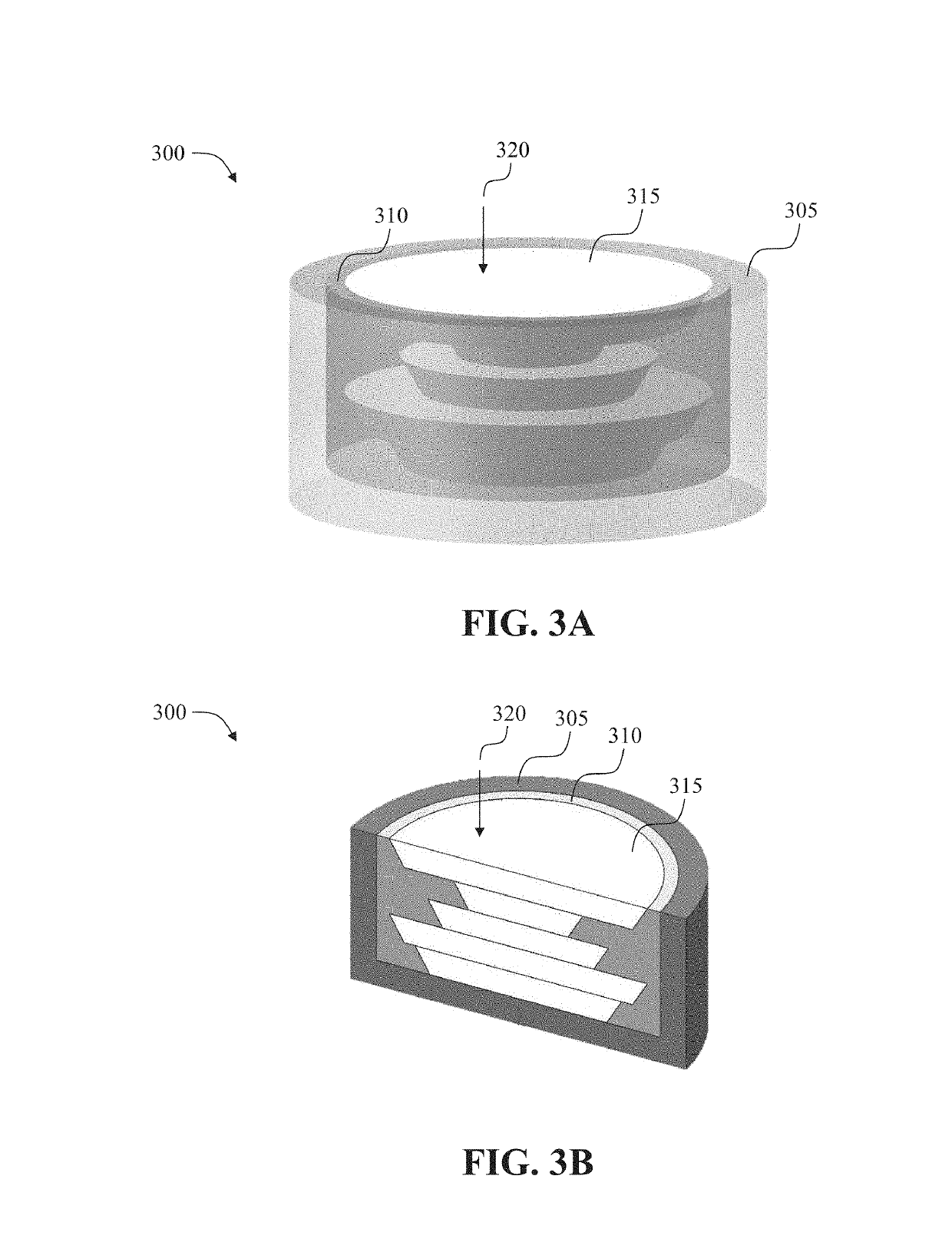
Dosage forms with desired release profiles and methods of designing and making thereof
ActiveUS10350822B1Organic active ingredientsPharmaceutical product form changeGel Dosage FormDesign methods
In some aspects, the present disclosure provides dosage forms, such as oral drug dosage forms, configured to provide a desired release profile, the dosage forms comprising a multi-layered structure comprising a plurality of layers of a first erodible material admixed with a compound (e.g., a drug) or a reagent, wherein the first erodible material is embedded in a second material not admixed with the compound (e.g., the drug) or the reagent. In other aspects, the present disclosure provides methods of designing, such as obtaining a thickness and / or surface area of a layer comprising an erodible material admixed with a compound (e.g., a drug) or a reagent, and / or amount of the compound (e.g., the drug) or the reagent admixed in the erodible material, and methods of making, such as three-dimensional printing, dosage forms configured to provide desired release profiles.
Owner:TRIASTEK INC
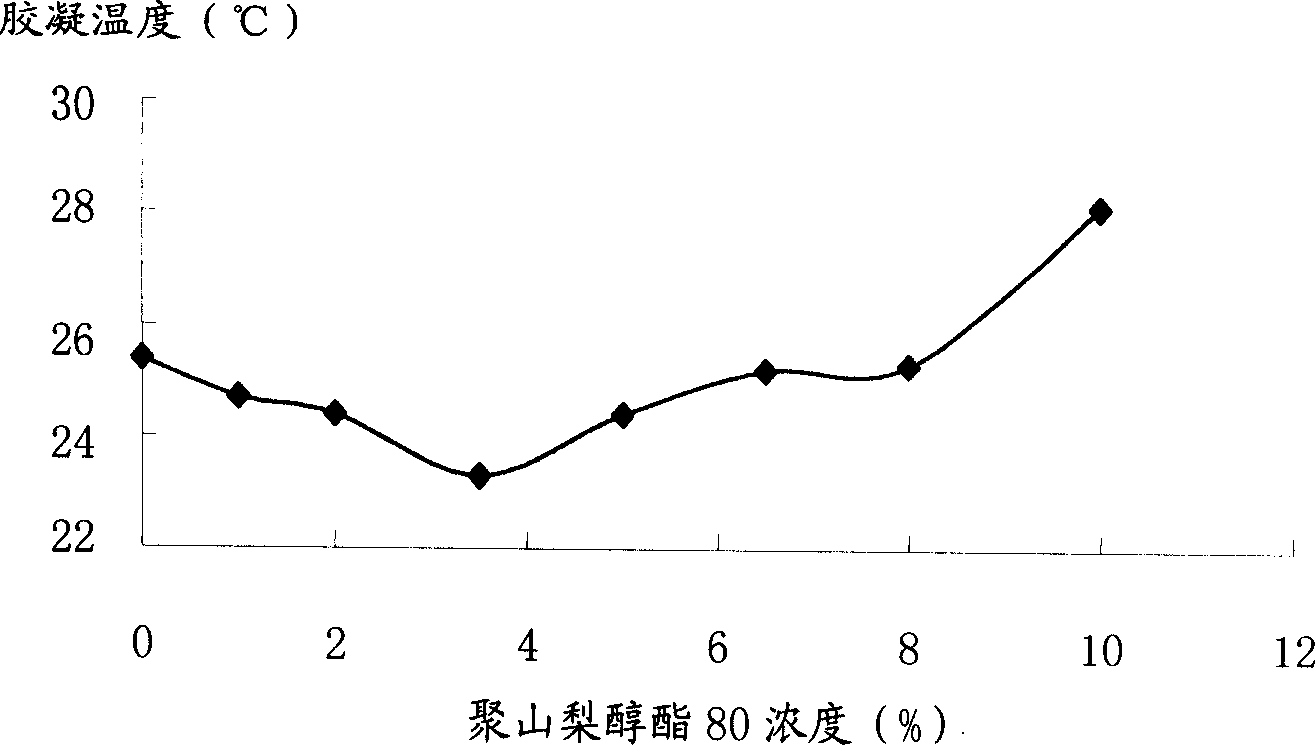
Temperature sensing instant type gel system
InactiveCN1869128AWide adjustable temperature rangeEasy to processPharmaceutical delivery mechanismPharmaceutical non-active ingredientsPharmaceutical drugEngineering
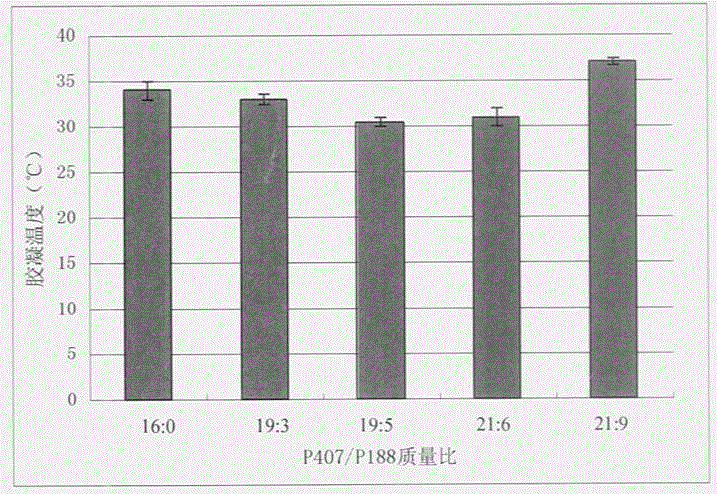
The invention discloses a temperature sensitive type instant molding gel system that includes poly-sorbate as gelatinizing temperature adjusting agent, and also could use poly-sorbate with other gelatinizing temperature adjusting agent. By adjusting the content of poly-sorbate, the adjustable range of gelling temperature would be expanded, and the solid content in system would be decreased, and the cost would be lowered. The invention could be used in normal medicine.
Owner:ZHEJIANG UNIV
Royal jelly acid containing antibacterial biological dressings as well as preparation method and application thereof
InactiveCN105536041AImprove antibacterial propertiesReasonable formulaAbsorbent padsBandagesAdditive ingredientBiological dressing
The invention discloses royal jelly acid containing antibacterial biological dressings as well as a preparation method and application thereof. One of the antibacterial biological dressings comprises the following ingredients in percentage by weight: 0.01-20% of royal jelly acid, 0.01-1% of a stabilizer, 1-5% of chitosan, alginate, polyving akohol or collagen and the balance of a matrix, wherein the stabilizer is alkyl trimethyl ammonium salt, multi-alkyl dimethyl ammonium salt or a betaine-type surfactant. Through the combination of royal jelly acid with one or more of chitosan, alginate, polyving akohol and collagen, a series of the antibacterial biological dressings of the solution / solvent type, the gel type, the cream type, the dressing type, the powder type and the patch type are prepared, can be used for treating infectious wounds, surgical wounds, burn wounds, scald wounds and the like, and are good in antibacterial property and broad in application prospect. The antibacterial biological dressings are reasonable in formula and convenient to prepare, and can control wound infection, keep the wounds wet and promote wound healing. More than one hundred of clinical cases prove that the effective rate of the antibacterial biological dressings reaches 95% or above.
Owner:常州陀谱生物医学技术有限公司
Preparation technology of a new integrated dosage form of Linggui Zhugan Decoction and its production method
InactiveCN102283895AWide distribution of targetsStrong slow and controlled release performanceSenses disorderNervous disorderNanocarriersBULK ACTIVE INGREDIENT
The invention relates to a preparation technology and a production method of a new integrated dosage form of Linggui Zhugan Decoction. The raw material composition of the active ingredients is as follows: 12 parts of poria cocos, 9 parts of cassia twig, 6 parts of atractylodes rhizome and 6 parts of roasted licorice. Its production process includes: supersonic jet pulverization, alcohol water extraction, ultrasonic pulverization and extraction, water decoction and concentration, supersonic spray drying, nano grinding, high pressure emulsification, nano particle preparation, etc. The invention pays attention to the advantages of multi-synergy and multi-targeting embodied by the combination technology of nano-carriers. After large-scale production, the production cost can be greatly reduced, the product quality can be greatly improved, and the targeting and sustained release of the drug can be strengthened. It can be administered both internally and externally, and can be taken in four time periods according to the meridian flow and the flow of blood in the human body. Film-forming agents and transdermal agents can also be prepared and pasted on different parts of the human body according to the meridian flow and the flow of human Qi and blood, and can be directly absorbed through the skin.
Owner:SUZHOU ZHIWEITANG BIOLOGICAL TECH +1
Pudilan children's antiviral oral pellet gel and preparation method thereof
ActiveCN104940301AGood curative effectImprove securityAerosol deliveryOintment deliveryAntibiosisMedicine
The purpose of the invention is to provide a Pudilan children's antiviral oral pellet gel and a preparation method thereof. The preparation method comprises the following steps: adopting a gel matrix, extracting Radix Scutellariae, Herba Taraxaci, Corydalis bungeana Turcz. and isatis root, preparing pellets by using extracted effective components, and uniformly dispersing the pellets in a gel. The prepared gel can be a solid gel, a semisolid gel or a xerogel. The method effectively covers the bad smell of medicines, solves the mouthfeel problem of the medicines, and allows the sweet oral pellet gel loved by children to be prepared, and the sweet and smooth mouthfeel of the oral pellet gel makes children easily take the medicine. Traditional Chinese medicines are combined with the gel dosage form to improve the children's administration long-term single dosage form, and the oral pellet gel has the efficacies of heat clearing, detoxification, antibiosis and inflammation prevention, and also has the advantages of high content of the effective component, small administrating dosage, stable preparation and good curative effect.
Owner:黑龙江童医生儿童生物制药有限公司
Highly porous, fast-disintegrating solid dosage form and its way of manufacturing comprising the preparation of a powder and a freezedrying step
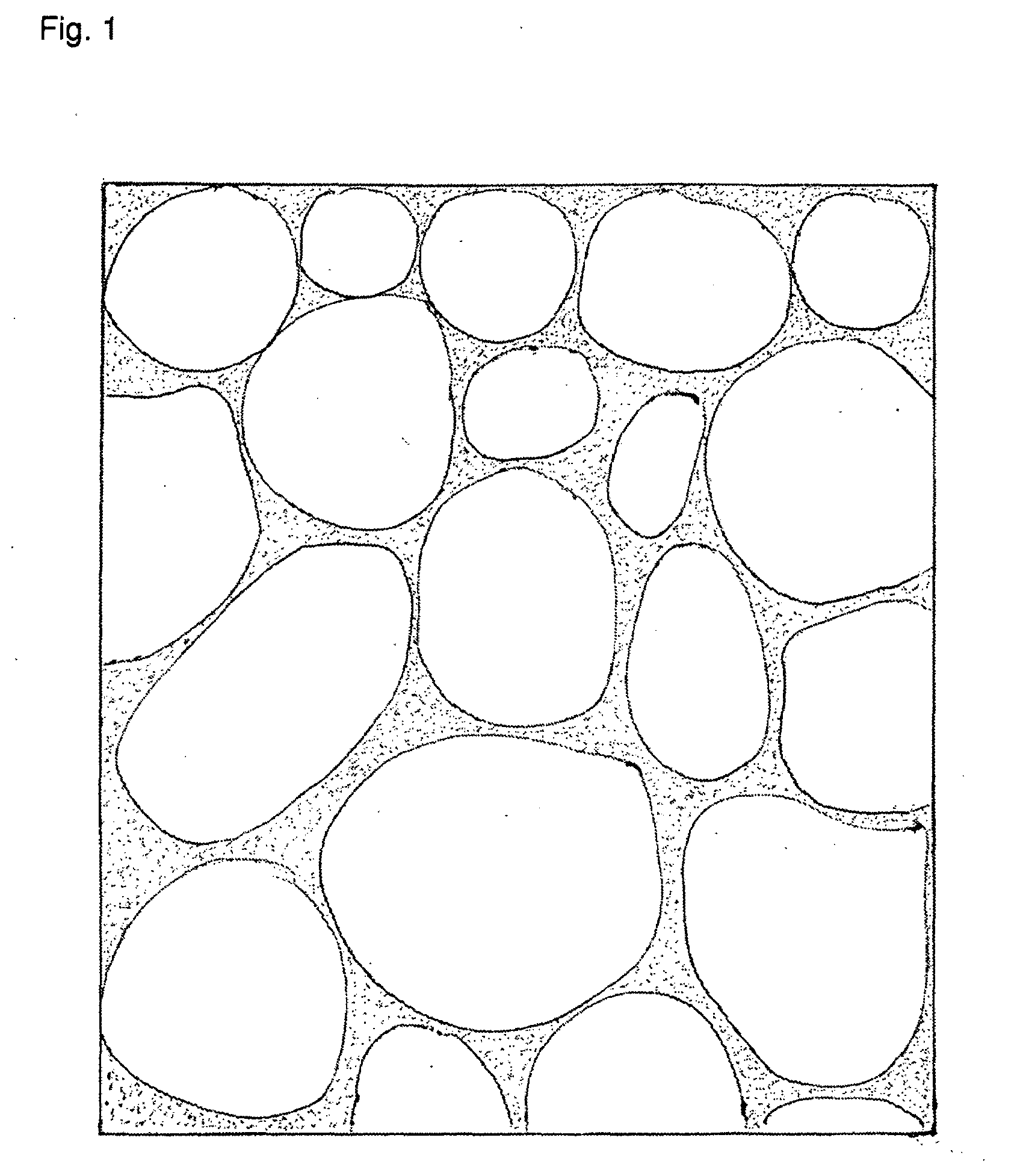
The invention relates to a method of manufacture of fast-disintegrating solid dosage forms, characterized in that one or more structure building components in mixed solid powder form are dosed into cavities of blister packs or moulds, the remaining components dissolved in water dosed and added to the powder to form a moistened, plasticized mass, frozen to below −20° C. , and the water sublimed in high vacuum. In this way solid dosage forms are obtainable with a similar porous structure as usually result from freeze-drying processes, but the process requires much less water, which means considerably less time and less energy.
Owner:PANTEC AG
Fast dispersing dosage forms free of gelatin
InactiveCN1337876ADissolve fastPowder deliveryPharmaceutical non-active ingredientsHigh concentrationFreeze-drying
The present invention relates to fast dispersing solid dosage forms that preferably dissolve in the oral cavity within sixty (60), more preferably within thirty (30), most preferably within ten (10) seconds. A novel feature of the solid dosage forms according to the invention reside in the fact that the composition is essentially free or absolutely free of mammalian gelatin. It has been discovered that the use of certain modified starches at concentrations from 20 to 90 % by weight of the solid dosage form prepares dosage forms that are mechanically and chemically stable and are able to deliver higher concentrations of an active ingredient than the heretofore utilized gelatin based fast dispersing solid dosage forms. Further, the solid dosage forms according to the invention are obtainable by removing a solvent, such as water, from a mixture comprising an active ingredient, a modified starch and a matrix forming agent via freeze drying.
Owner:R.P.斯克尔公司
Gel matrix
ActiveCN103211753AStable and uniform storageFacilitated releaseAerosol deliveryOintment deliverySide effectAdditive ingredient
The invention provides a gel matrix and a preparation method thereof, and the gel matrix is used for preparation of gel dosage form drugs. The gel matrix provided by the invention includes: 0.5-10% of gel, 0.1%-10% of a tissue stabilizer, 0.1%-10% of a tissue repair agent, 0.5%-5% of a transdermal enhancer, 1%-10% of a non-water solvent with concentration higher than 90% and water, or a non-water solvent with concentration not more than 50%. The gel matrix provided by the invention has certain leveling property (uniformity), flexibility and moisture retention, so as to reduce the ''parching'' phenomenon. The gel matrix provided by the invention has strong ability of bearing and releasing drugs to satisfy quantitative addition of medicinal ingredient and guarantee stability and uniformity of drug storage and drug concentration in a course of treatment. The gel matrix provided by the invention can improve release rate, percutaneous absorption rate and safe treatment of the drug. The invention can increase tissue compatibility of the preparation, properly prolong action time and application frequency, so as to increase effectiveness and reduce the occurrence of local side effects.
Owner:北京中美联医学科学研究院有限公司
Silicon-based wound repair hydrogel and preparation method thereof
The invention discloses a silicon-based wound repair hydrogel which is prepared from the following raw materials: 0.8-1.5g of carbomer, 40-60mL of glycerinum, 45-70mL of propylene glycol, 0.3-1.2g of gelatin, 6-16g of bioactive glass and 0.6-1.4g of pearl powder. A pearl powder component which contains calcium carbonate and various amino acids and is capable of providing local tissue of a wound with nutrients is added on the basis of a silicon-based wound repair mateiral, namely bioactive glass as a main repair material; meanwhile, the dosage form of the hydrogel is prepared under the condition of not adding water, so that the silicon-based wound repair material is prevented from degenerating in water, the dosage form has the characteristic that the hydrogel is painted for protecting the wound, and the silicon-based wound repair hydrogel is capable of keeping the wound moist, creating a low-oxygen and slight-acid environment, accelerating wound healing, absorbing seepage and cleaning the wound in an autolytic manner.
Owner:江西虹景天药业有限公司 +1
Solid dosage form comprising solid dispersion
ActiveUS8343548B2Dissolve fastGood disintegrationPowder deliverySolution deliverySolubilityLow-substituted hydroxypropylcellulose
Owner:SHIN ETSU CHEM CO LTD
Swellable dosage form comprising gellan gum
ActiveUS20120039969A1Increase intakeEasy to doCosmetic preparationsHeavy metal active ingredientsParticulatesGellan gum
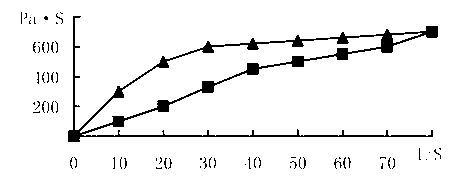
A novel dosage form. The dosage form is presented in particulate form and before oral ingestion the particulate material is subjected to an aqueous medium, whereby it is converted to a semi-solid form by swelling or gelling of one or more of the components, especially of a gellan gum, of the particulate matter. The invention also relates to a vehicle for oral administration of one or more active substances, the vehicle comprising a gellan gum arranged in a configuration allowing optimal water diffusion so that upon addition of a predetermined amount of an aqueous medium, without the necessity of applying shear forces or other mixing forces, within a time period of 5 minutes or less swells and / or gels and the texture of the swelled vehicle being similar to that of a soft pudding and having a viscosity of at least about 10,000 cps as measured by a Brookfield Viscometer with a #4 LV spindle at 6 rpm and at 20-25° C. In one embodiment of the invention, the particulate matter can be molded into a desired shape or pressed onto a dispensing unit such as a spoon.
Owner:ADARE PHARM INC
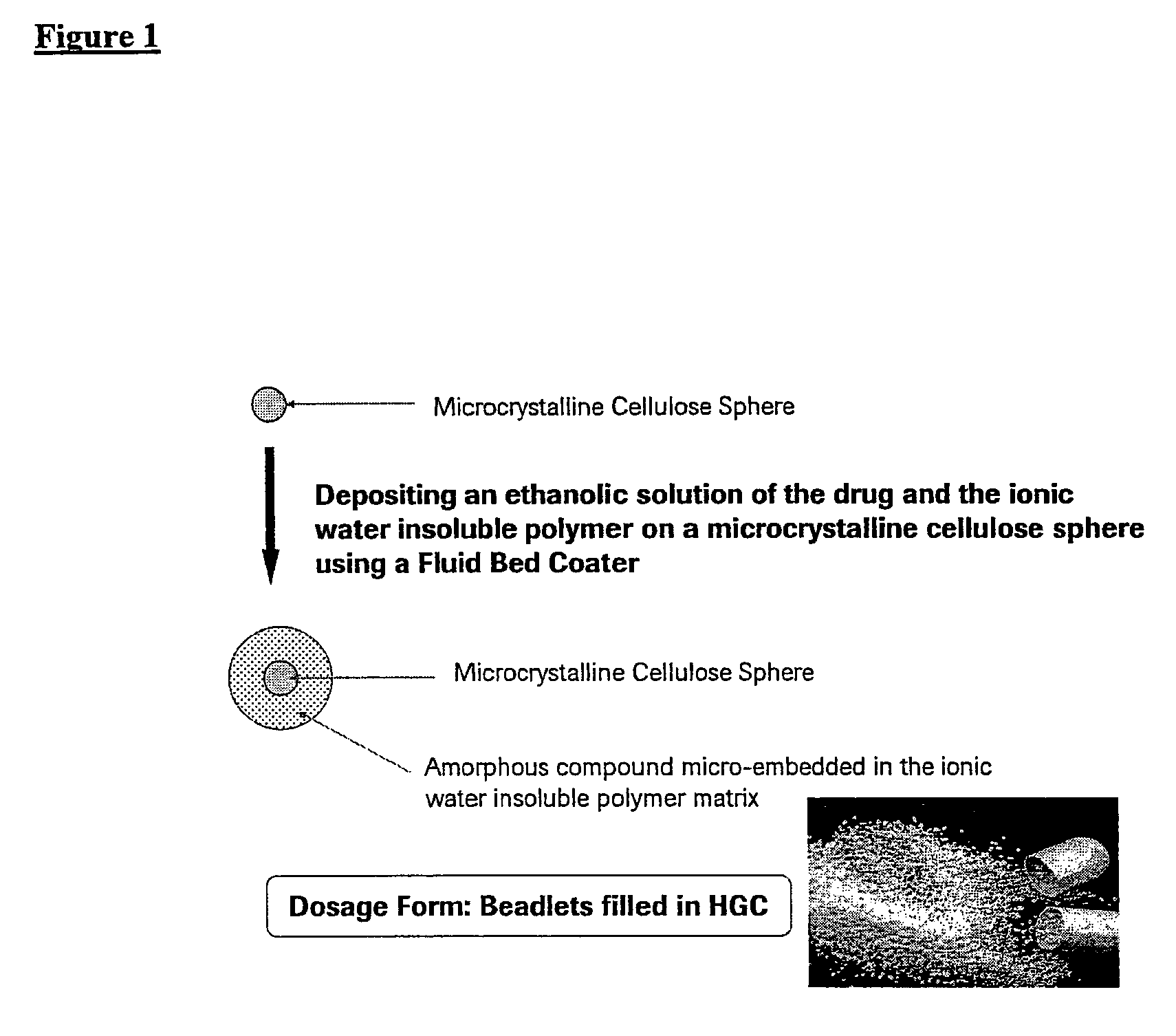
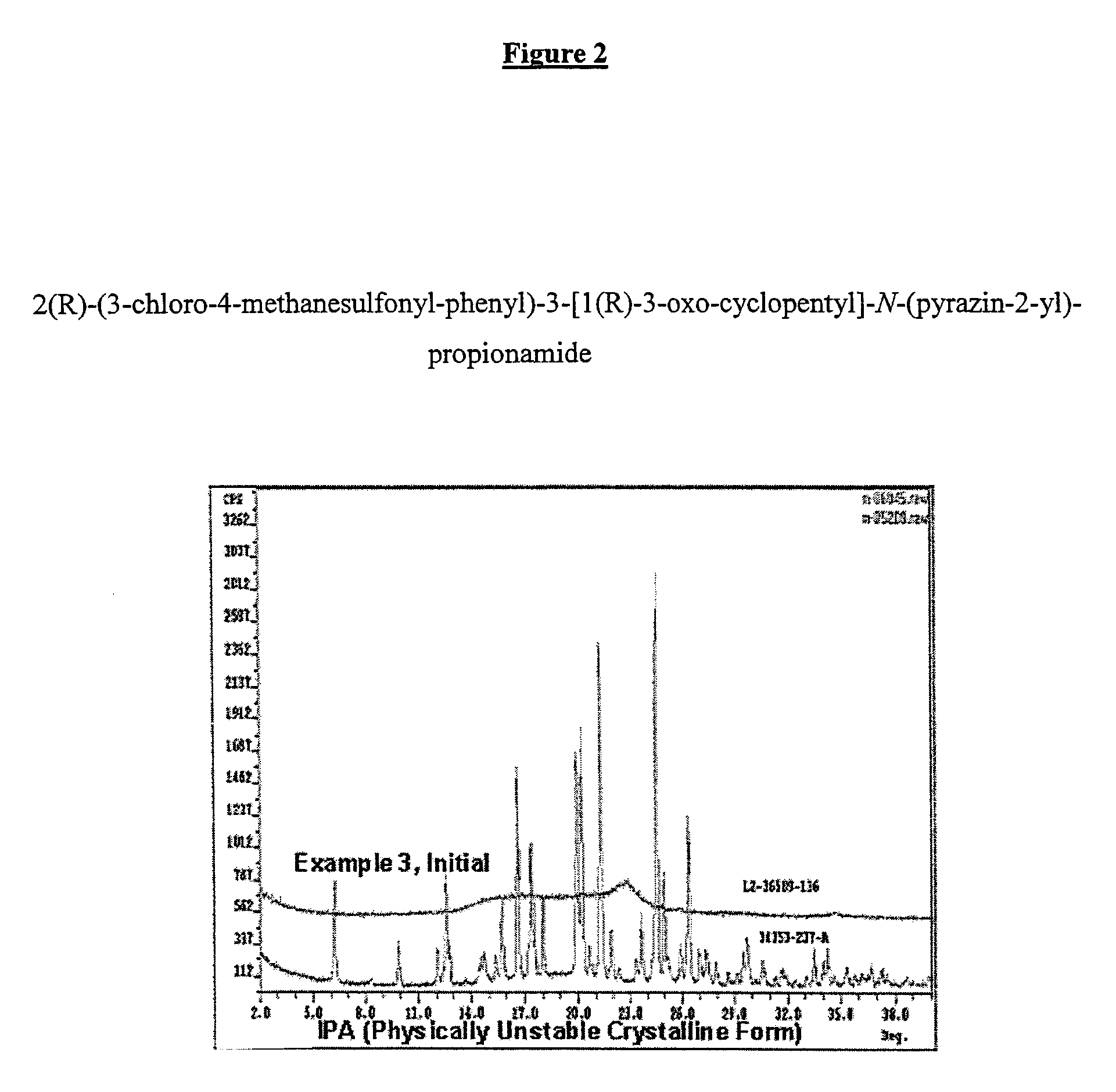
Pharmaceutical Solid Dosage Forms Comprising Amorphous Compounds Micro-Embedded in Ionic Water-Insoluble Polymers
The present invention provides novel pharmaceutical solid dosage forms for oral administration comprising a therapeutically effective amount of an unstable crystalline form or an amorphous form of a therapeutically effective compound micro-embedded into an ionic water-insoluble polymer. The therapeutically effective compounds, which have a tendency to gel, are micro-embedded into an ionic water-insoluble polymer matrix to provide a dosage form having rapid, reproducible, and complete dissolution profiles. These novel solid pharmaceutical dosage forms are useful in the treatment or control of a number of diseases. The present invention also provides a method for treating a disease comprising administering to a subject, in need thereof, a therapeutically effective amount of the novel solid pharmaceutical dosage form. The present invention further provides a method for preparing the pharmaceutical dosage forms.
Owner:ALBANO ANTONIO AQUINO +4
Solid dosage form comprising solid dispersion and method for producing the same
InactiveCN101125204AImprove solubilityPharmaceutical non-active ingredientsGranular deliverySolubilityLow-substituted hydroxypropylcellulose
Provided are a solid dosage form comprising an enteric solid dispersion that allows a drug in the preparation to be rapidly dissolved without compromising the solubility of the solid dispersion, and a method for producing the same. More specifically, provided is a solid dosage form comprising an enteric solid dispersion comprising a poorly soluble drug, an enteric polymer and a disintegrant, wherein the disintegrant is low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 [mu]m and a specific surface area measured by BET method of at least 1.0 m 2 / g. Moreover, provided is a method for producing a solid dosage form comprising an enteric solid dispersion, the method comprising steps of: spraying an enteric polymer solution in which a poorly soluble drug has been dispersed or dissolved, on a powder of low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 [mu] m and a specific surface area measured by BET method of at least 1.0 m 2 / g and serving as a disintegrant; and granulating the resultant; and drying.
Owner:SHIN ETSU CHEM IND CO LTD
Solid dosage form comprising solid dispersion and method for producing the same
ActiveUS20080038339A1Improve solubilityDissolve fastBiocidePowder deliverySolubilityLow-substituted hydroxypropylcellulose
Provided are a solid dosage form comprising a solid dispersion that allows a drug in the preparation to be rapidly dissolved without compromising the solubility of the solid dispersion, and a method for producing the same. More specifically, provided is a solid dosage form comprising a solid dispersion, the dispersion comprising: a poorly soluble drug, a water-soluble polymer and a disintegrant, wherein the disintegrant is low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g. Moreover, provided is a method for producing a solid dosage form comprising a solid dispersion, the method comprising steps of: spraying a water-soluble polymer solution in which a poorly soluble drug has been dispersed or dissolved, on a powder of low-substituted hydroxypropylcellulose having an average particle size of 10 to 100 μm and a specific surface area measured by BET method of at least 1.0 m2 / g and serving as a disintegrant and granulating the resultant; and drying.
Owner:SHIN ETSU CHEM IND CO LTD
Dosage form having a saccharide matrix
InactiveCN1758899AHigh mechanical strengthPowder deliveryAllergen ingredientsGel Dosage FormSorbitol
The present invention provides a non-compressed fast-dispersing solid dosage form suitable for oromucosal administration of a pharmaceutically active substance comprising (a) a first matrix forming agent in the form of maltodextrin having a dextrose equivalent (DE) of between 1 and 20,(b) a second matrix forming agent in the form of sorbitol, and (c) the active substance.
Owner:ALK ABELLO SA
External use medicine composition and preparing method thereof
InactiveCN104338118ASmooth appearanceDelicate appearancePeptide/protein ingredientsDermatological disorderEffective treatmentAstressin-B
The invention belongs to the technical field of medicine compositions, and discloses an external use medicine composition used for treating alopecia. The external use medicine composition comprises annular adrenocorticotrophic hormone release factor (CRF) antagonism peptide astressin B in effective treatment amount or acceptable salt on the pharmacy. The external use medicine composition is in a gel dosage form preferably and suitable for clinic use and industrialization production. In addition, the invention further discloses a method for preparing the external use medicine composition and application of the astressin B in effective treatment amount or the acceptable salt on the pharmacy to preparing the external use medicine composition used for treating the alopecia. According to the external use medicine composition, the problem of the alopecia for a long time can be effectively treated; and the external use medicine composition is safe, reliable, free of obvious skin irritants and free of sensitization.
Owner:HYBIO PHARMA
Hemostatic antibacterial healing-promoting medical dressing containing lamiophlomis rotata iridoid glycoside composite activated carbon fibers (ACF) as well as preparation method and application of medical dressing
InactiveCN107158446ALarge specific surface areaLarge specific surface area, large effective adsorption capacityPharmaceutical delivery mechanismAbsorbent padsFiberPolyvinyl alcohol
The invention discloses a hemostatic antibacterial healing-promoting medical dressing containing lamiophlomis rotata iridoid glycoside active ingredient composite medical ACF and belongs to the technical field of preparation of medical dressings. The medical dressing is composed of the following components in percentage by weight: 1-20% of lamiophlomis rotata iridoid glycosides, 0.01-1% of hexadecyl trimethyl ammonium chloride, 1-5% of polyvinyl alcohol or collagen and the balance of a medical ACF matrix. The lamiophlomis rotata iridoid glycoside active ingredients and polyvinyl alcohol or collagen and the like are combined to be prepared into a solution dosage form and a gel dosage form, and the dosage forms are compounded with medical ACFs so as to prepare a series of external medical dressings. By utilizing the strong effects of the ACF for adsorbing wound exudates, activating a clotting mechanism and accelerating hemostasis, the 'synergistic effect' between the two is achieved, and the medical dressing can be used for treating infectious wounds, surgical trauma bleeding and the like, has excellent hemostatic antibacterial healing-promoting effects and also has wide application prospects. The medical dressing disclosed by the invention is reasonable in formula and convenient in preparation and has the effects of controlling the wound infection and promoting healing of the wound. The hemostasis models for wounds at artery and ventral aorta in ears of large-ear white rabbits and human-mouse livers prove that the effective rate reaches 95% or higher.
Owner:CHINA PHARM UNIV
Hydrogel-driven drug dosage form
InactiveCN1461212AFast transferLow residual drugOrganic active ingredientsInorganic non-active ingredientsWater insolubleGel Dosage Form
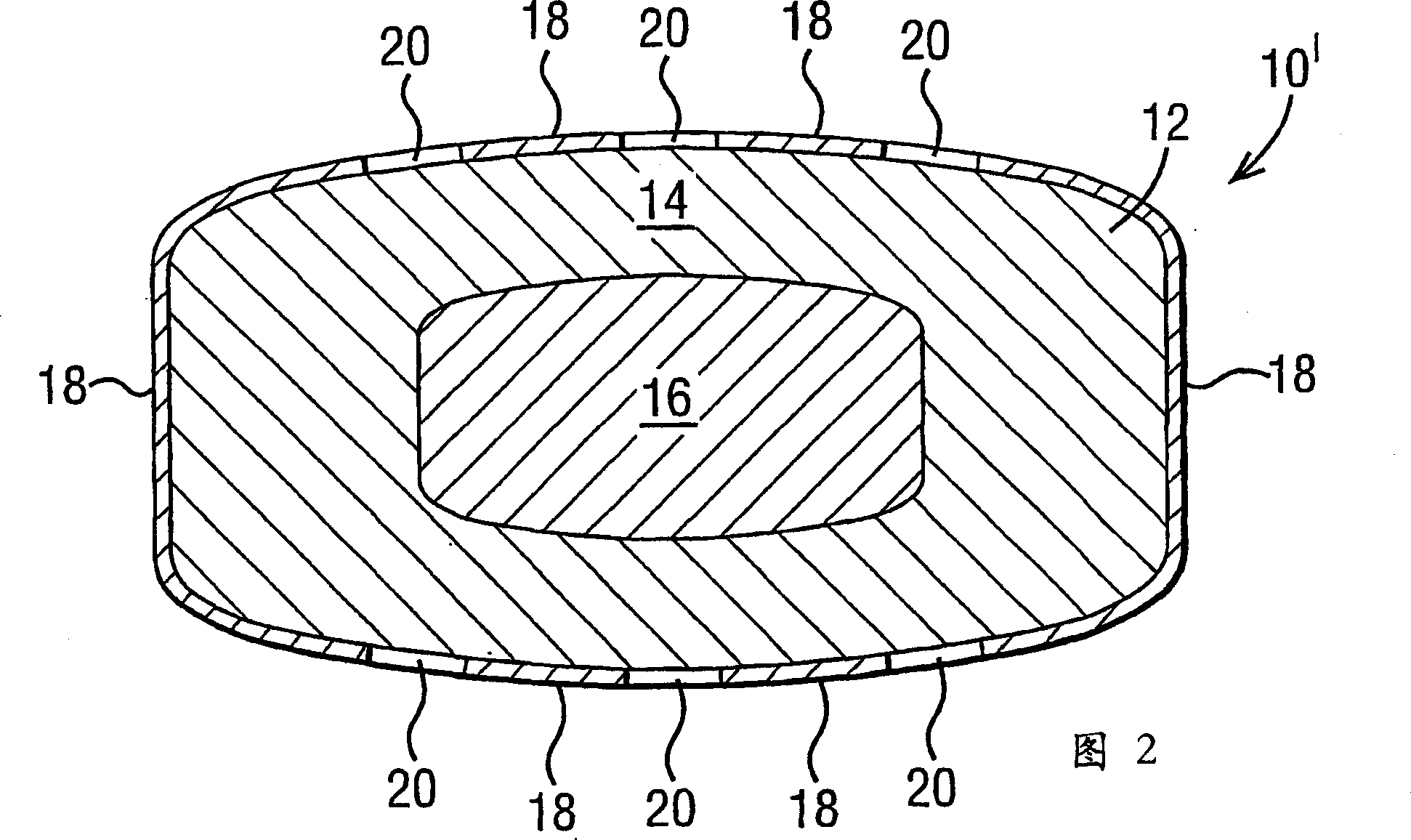
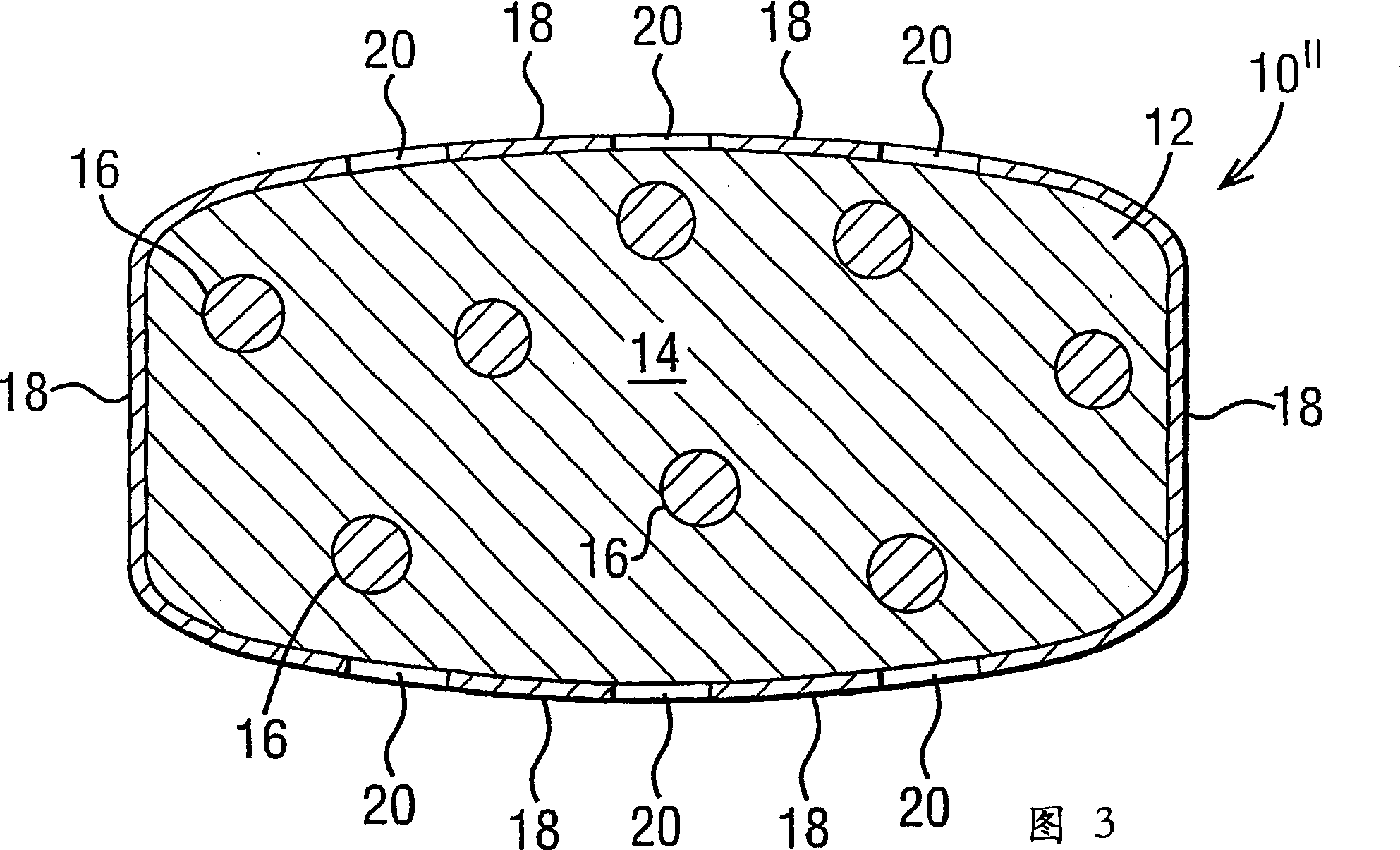
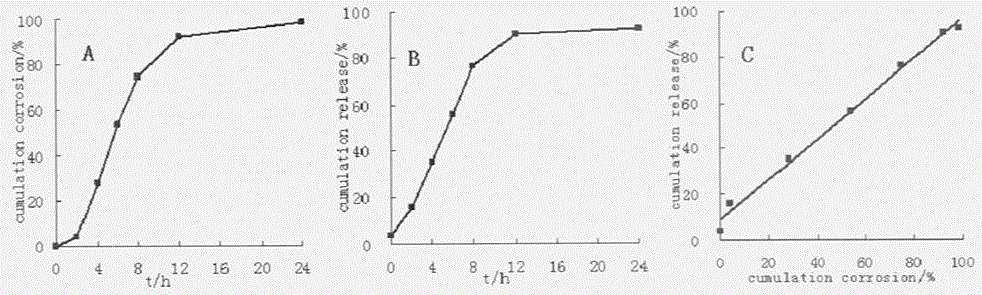
A controlled release dosage form has a coated core with the core comprising a drug-containing composition and a water-swellable composition, each occupying separate regions within the core. The coating around the core is water-permeable, water-insoluble and has at least one delivery port therethrough. A variety of geometric arrangements are disclosed.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Injectable temperature-sensitive gel preparation for treating acute pancreatitis
InactiveCN105342984ASmall systemic side effectsSimple preparation processOrganic active ingredientsPeptide/protein ingredientsAcute pancreatitisPolyvinyl alcohol
Critical conditions of acute pancreatitis have high mortality. Trypsinization Theory is one of important pathogeneses of acute pancreatitis. Pancreatin inhibitor drugs, such as gabexate mesylate, ulinastatin and aprotinin, are selectively clinically applied against the mechanism. The routine administration mode of the drugs is intravenous injection, and has certain disadvantages. The invention provides an injectable temperature-sensitive gel preparation for treating acute pancreatitis. The dosage form of the above gel comprises a poloxamer polymer, a polylactide-co-glycolide / polyethylene glycol block copolymer, a polycaprolactam / polyethylene glycol block copolymer, a chitosan / beta-sodium glycerophosphate system, a chitosan / polyvinyl alcohol system or a chitosan-sodium bicarbonate system, or an arbitrary combination thereof. The gel has a reverse gelling characteristic, is a liquid at a low temperature, and converts to a semisolid state at a human body temperature. The invention also provides a preparation method of the injectable temperature-sensitive gel preparation for treating acute pancreatitis.
Owner:GENERAL HOSPITAL OF PLA
Method of making dosage forms comprising polymeric compositions
A dosage form comprises: (a) at least one active ingredient; (b) a core having an outer surface; and (c) a shell which resides upon at least a portion of the core outer surface, wherein at least a portion of the shell is semipermeable, such that the liquid medium diffuses through the semipermeable shell or shell portion to the core due to osmosis. The shell also provides for delivery of the active ingredient to a liquid medium outside the shell after contacting of the dosage form with the liquid medium. The dosage form delivers one or more active ingredients in a controlled manner upon contacting of the dosage form with a liquid medium. The dosage form may be employed to provide a burst release of the active ingredient, or to provide release of the active ingredient at an ascending release rate over an extended time period upon contacting of the dosage form with a liquid medium. At least a portion of the shell may be comprised of a polymeric composition containing film former, gelling agents, which can be dissolved in a multisolvent system comprised of water and an organic solvent.
Owner:HUANG HAI YONG +2
Antiviral hydrogel as well as preparation method and application thereof
ActiveCN111821254AGood biocompatibilityStrong antiviral activityAerosol deliveryDigestive systemAntiviral drugPharmaceutical drug
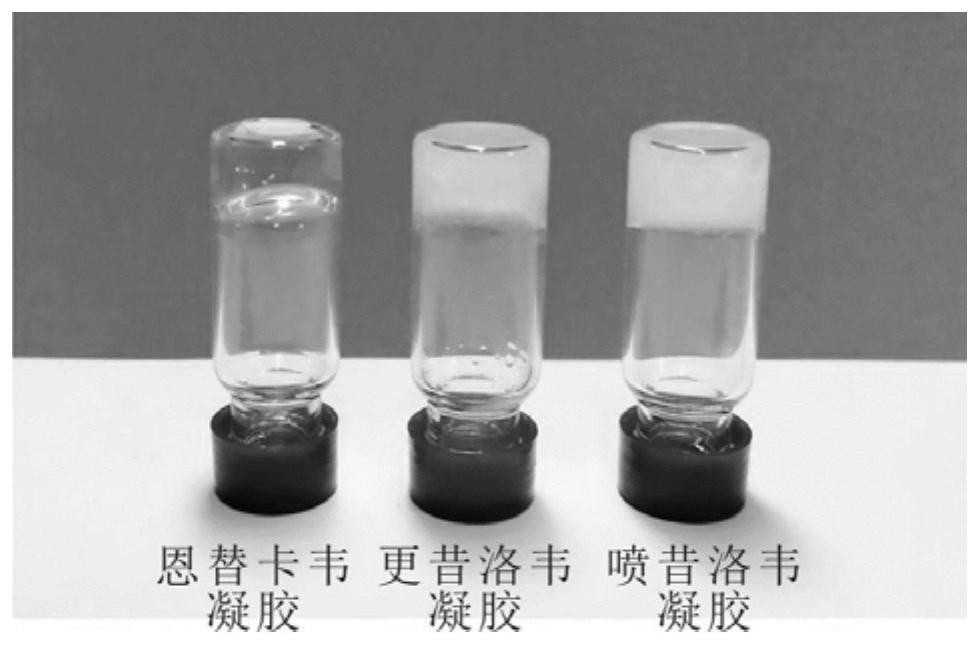
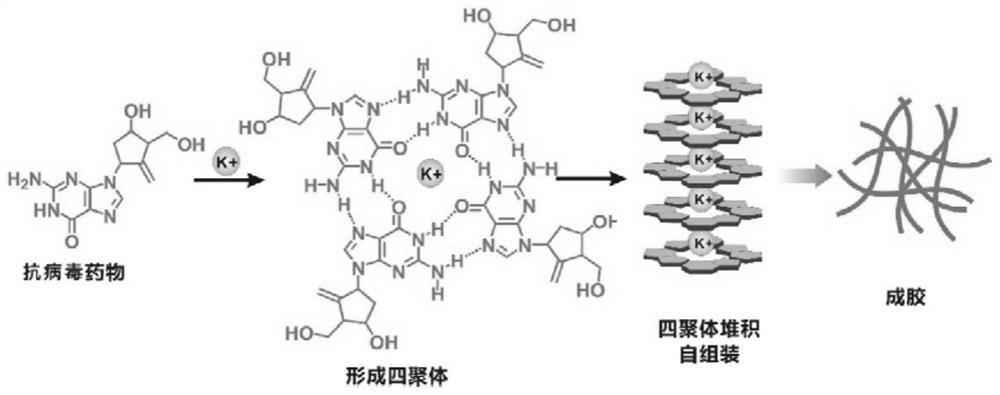
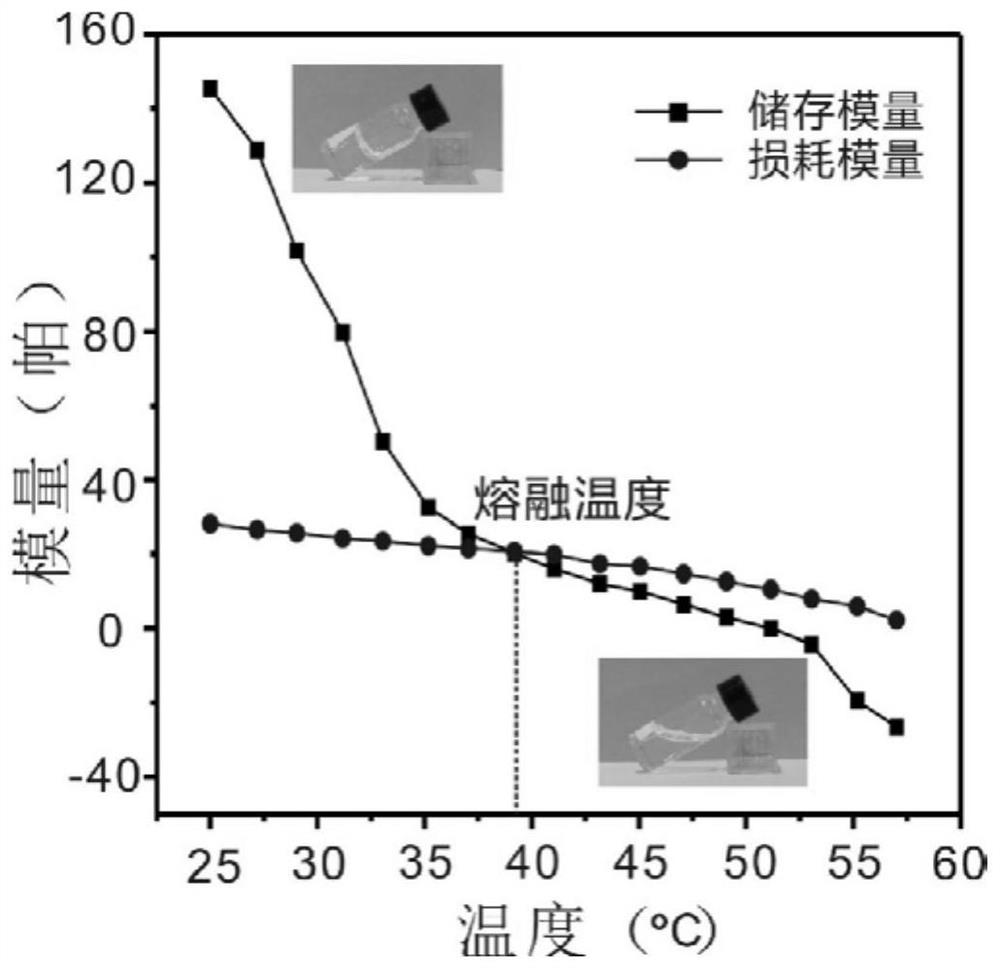
The invention discloses an antiviral hydrogel as well as a preparation method and application thereof. The hydrogel is directly formed by stacking and assembling nucleoside antiviral drugs layer by layer in the presence of metal ions. The antiviral hydrogel provided by the invention has antiviral activity equivalent to that of a free drug, can be prepared into a gel dosage form, and has a clinicalantiviral infection treatment application prospect.
Owner:EAST CHINA NORMAL UNIV
Highly porous, fast-disintegrating solid dosage form and its way of manufacturing comprising the preparation of a powder and a freezedrying step
The invention relates to a method of manufacture of fast-disintegrating solid dosage forms, characterized in that one or more structure building components in mixed solid powder form are dosed into cavities of blister packs or moulds, the remaining components dissolved in water dosed and added to the powder to form a moistened, plasticized mass, frozen to below −20° C., and the water sublimed in high vacuum. In this way solid dosage forms are obtained with a similar porous structure as usually result from freeze drying processes, but the process requires much less water, which means considerably less time and less energy.
Owner:PANTEC AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com