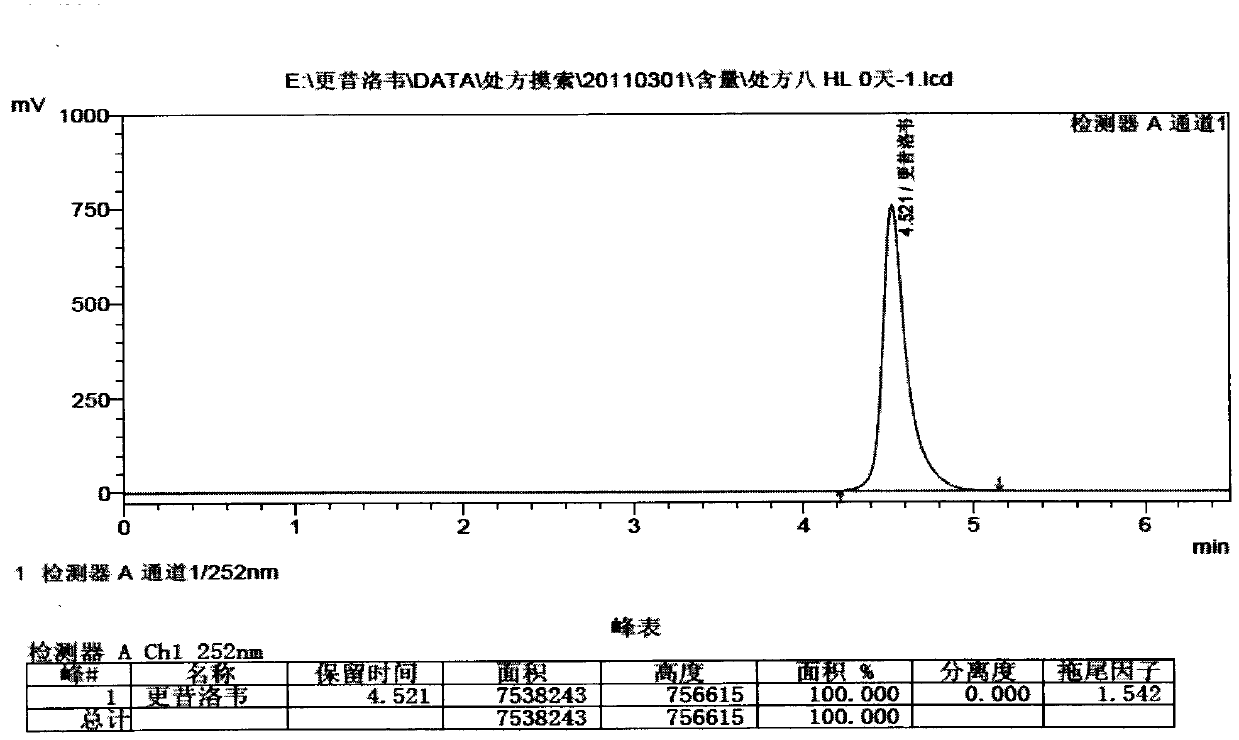
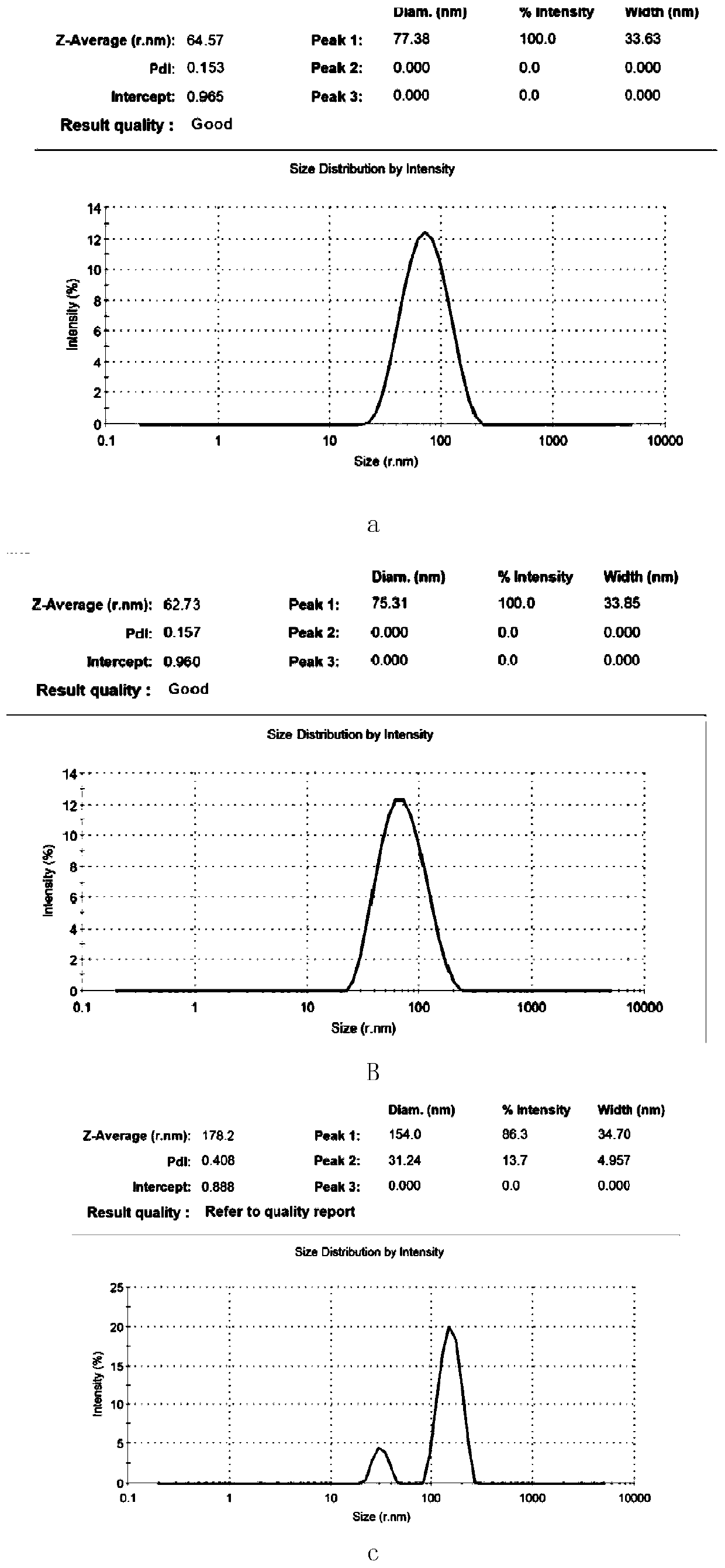
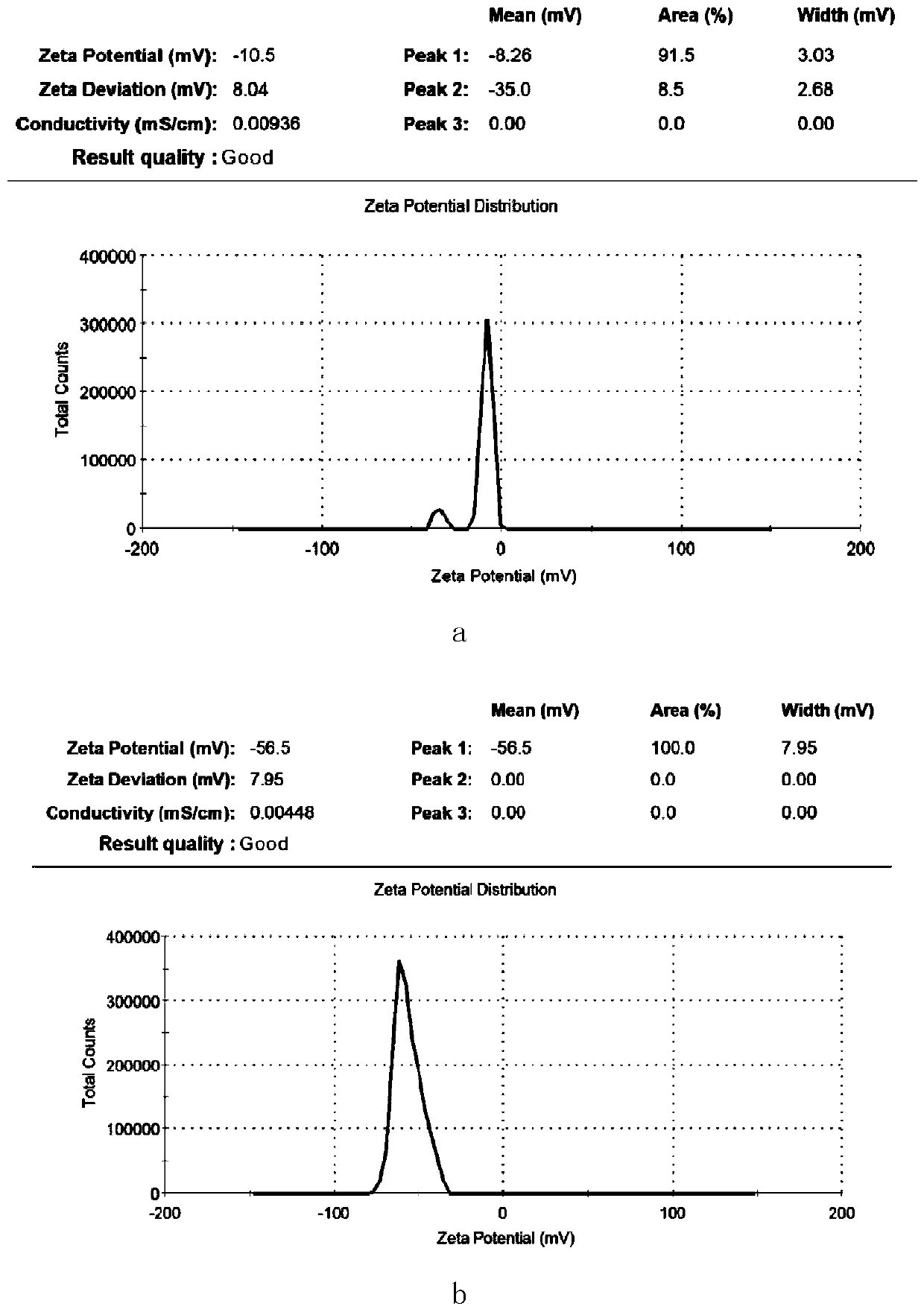
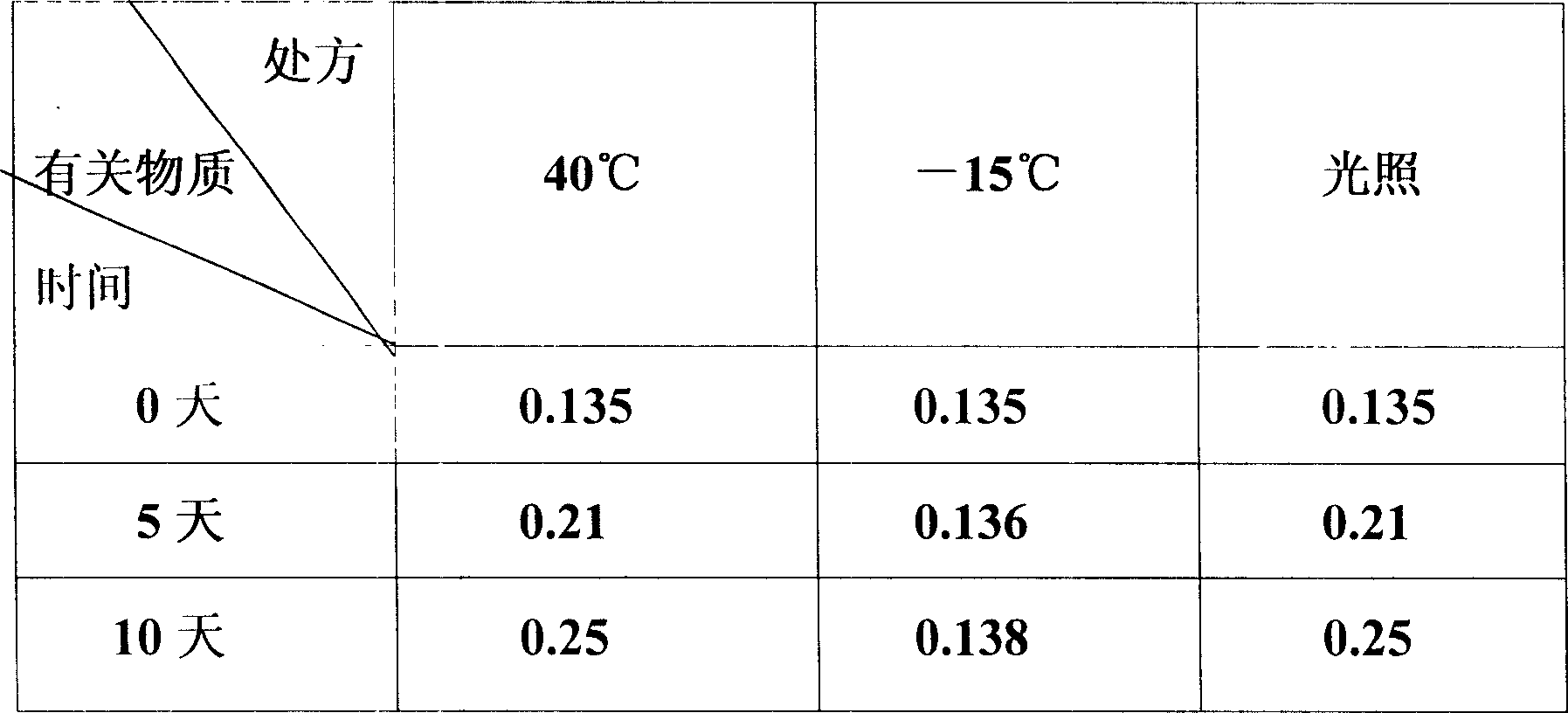
Patents
Literature
45results about How to "Small systemic side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel application of eye medicine wrapped with erythrocyte membrane
ActiveCN103550223AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderDiseaseErythrocyte membrane
The invention provides application of an eye medicine wrapped with an erythrocyte membrane in preparation of an eye preparation, and also provides the eye preparation. The preparation is in a form of biodegradable nano particles wrapped with the erythrocyte membrane. The preparation has the advantages that the shortcomings of the traditional eye drops that partial removal is quickly realized, the bioavailability is low, the eye drops are absorbed by the whole body, and the side effect is difficult to avoid, are overcome; the time of the medicine standing in eyes is obviously prolonged; the controlled-released administration of the medicine is realized. Therefore, the bioavailability of the medicine is improved, the side effect on the whole body is reduced, and a new hope is brought to clinical ophthalmology on treatment of intraocular diseases.
Owner:GUANGZHOU KANGRUI BIOLOGICAL PHARMA TECH CO LTD
Pharmaceutical composition for treating ulcerative colitis, its preparation method and use
InactiveCN1768780ATake a small doseEasy to takeDigestive systemSuppositories deliverySophora RootMedicine
The invention discloses a pharmaceutical composition for treating ulcerative colitis, which is prepared from astragalus root and flavescent sophora root as the raw material. the invention also provides the method for preparation and use of the pharmaceutical composition.
Owner:杨明
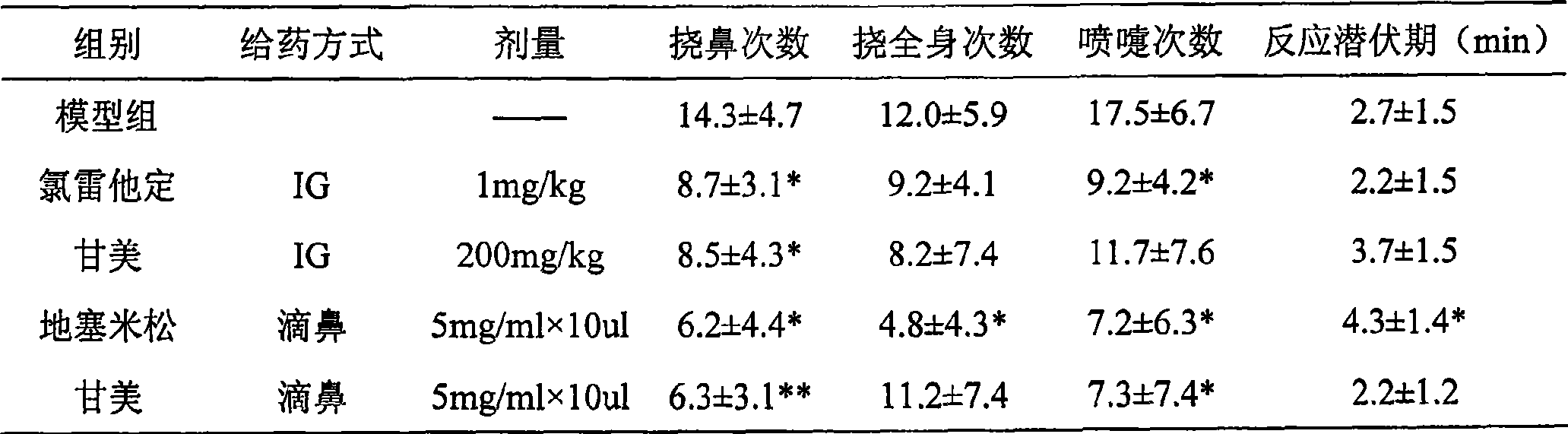
Use of iso-glycyrrhizic acid and salt thereof in treating allergic rhinitis
ActiveCN101396368ASymptoms improvedGood treatment effectOrganic active ingredientsAerosol deliveryActive componentNasal spray
The invention relates to application of iso-glycyrrhizic acid or salt in a drug for remedying allergic rhinitis and the preparation of nasal drop and nasal spray preparation with the iso-glycyrrhizic acid or salt as an active component.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Rupatadine fumarate eye drops and preparation method thereof
ActiveCN101926762AEasy to prepareSignificant effectOrganic active ingredientsSenses disorderDiseaseSide effect
The invention relates to rupatadine fumarate eye drops. The eye drops are prepared from rupatadine fumarate serving as a primary medicament, and a thickening agent, buffer salt, an isotonic agent, a bacteriostatic agent and a solubilizer serving as auxiliary materials. A preparation method A comprises the following steps of: dissolving the thickening agent by using a certain amount of water, and dissolving the primary medicament, the buffer salt, the isotonic agent, the bacteriostatic agent and the solubilizer by using a certain amount of water for injection; and mixing the two kinds of solution, and adding a proper amount of water for injection to reach the needed concentration. A preparation method B comprises the following steps of: stirring the thickening agent, the rupatadine fumarate, the buffer salt, the bacteriostatic agent, the isotonic agent, the solubilizer and the water for injection for a long time until the solution is clarified; and supplying the water for injection to fix the volume. The rupatadine fumarate eye drops have the advantages of simple preparation method and use for treating ocular surface allergic diseases, and have the characteristics of obvious curative effect, no irritation, no toxic or side effect, high stability and the like.
Owner:HAICHANG CONTACT LENSES +1
Oral colon target preparation for treating colitis ulcerativa and its making method
InactiveCN1853655ANovel dosage formEasy to takeAnthropod material medical ingredientsDigestive systemPeriplanetaUlcerative colitis
An orally taken target medicine for treating the ulcerative colitis with high curative effect and low dosage is prepared from the extract of American periplaneta. Its preparing process is also disclosed.
Owner:杨明
Alprostadil nano granule formulation and preparation thereof
InactiveCN101322712AReduce chance of regroupingOvercoming chemistryPowder deliveryOrganic active ingredientsDiseasePulmonary vasculature
The invention relates to an alprostadil nanoparticle preparation and a preparation method thereof. The preparation is essentially used for transdermal administration and used for treating diseases such as diabetic ulcer, ischemia of extremity end, burn and the like, and belongs to the technical field of medicine. The preparation consists of the raw materials by the following weight ratio that alprostadil:lipid component: emulsifier: excipient matrix: water is 0.01-0.1:0.5-10:0.5-5:550-950:5. The alprostadil nanoparticle preparation has the advantages of overcoming the chemical and physiological instability of the alprostadil, strengthening in vitro stability, reducing degradation of the alprostadil caused by inactivation effect of in vivo pulmonary circulation, enhancing concentration of the preparation on the local affected parts, being more easy to aggregate at the affected part, sustained release and long effect, and reducing irritation of the preparation.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Method of fixing and expressing physiologically active substance
InactiveUS20100329993A1Safe and effective retentionHigh feasibilityBiocideOrganic active ingredientsMedicineTherapeutic effect
The present invention provides methods for retaining and expressing physiologically active substances in a target tissue-specific-manner, by administering the physiologically active substances to target submucous tissue. Specifically, the present inventors demonstrated that, when physiologically active substances were directly administered into submucous tissues without using a carrier, the physiologically active substances were effectively and safely retained at the administration sites over long periods without loss and diffusion, and produced the effect acting in a reservoir-like fashion. The physiologically active substances administered as described above were demonstrated to produce the therapeutic effect without having an influence on organs other than the administered organ.
Owner:STELIC INST OF REGENERATIVE MEDICINE STELIC INST +1
Medicament coating material and preparation method
InactiveCN101934093AEasy to synthesizeInhibit aggregationCoatingsProsthesisBiocompatibility TestingVascular tissue engineering
The invention relates to the field of vascular tissue engineering, in particular to a medicament coating material which can be used in vascular tissue engineering and intervened in a stent and a preparation method. The coating material has the characteristics of degradability and an effect on promoting the growth of endothelial cells and inhibiting platelet adhesion. The coating material takes a polymer material which has good biocompatibility as a medicament carrier, and takes an effective extracts such as ferulic acid and derivatives thereof of a traditional Chinese medicament angelica sinensis. The preparation method has the following processes of: fully dissolving the medicament carrier and a medicament with a solvent; and using after mixing uniformly by ultrasonic sound. The medicament coating material can be prepared by a solution pouring method, wherein uniformly dissolved solution is poured in glass ware; volatilizing the solvent for 24 hours at the temperature of between 20 and 25 DEG C in vacuum to form a film; continuing heating for volatilizing the solvent thoroughly for 48 hours; and sterilizing for later use. The coating material has the effects on inhibiting the platelet adhesion and prompting the growth of endothelial cells, and can be used in the field of vascular tissue engineering and intervened in a degradable medicament coating on a stent surface.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
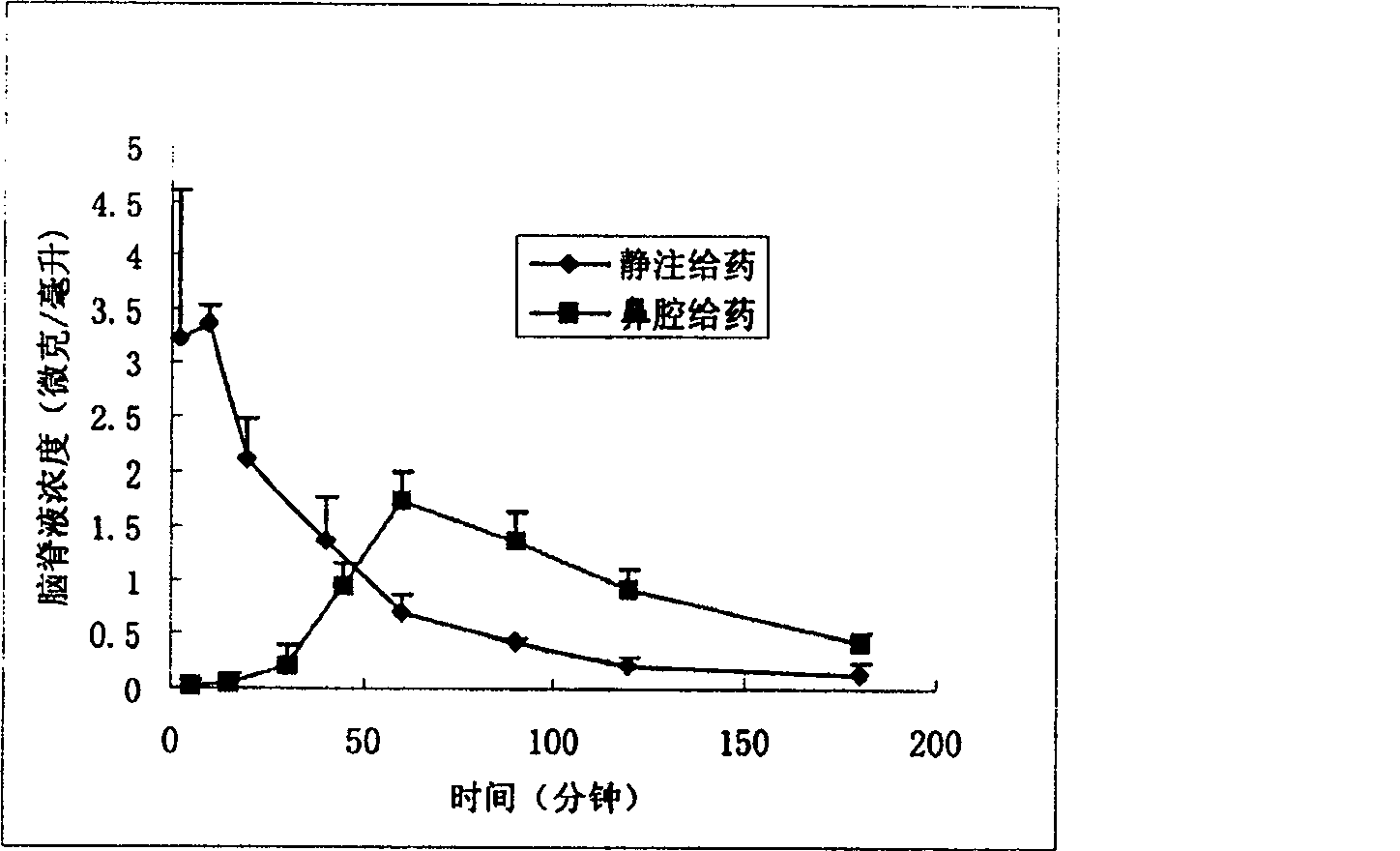
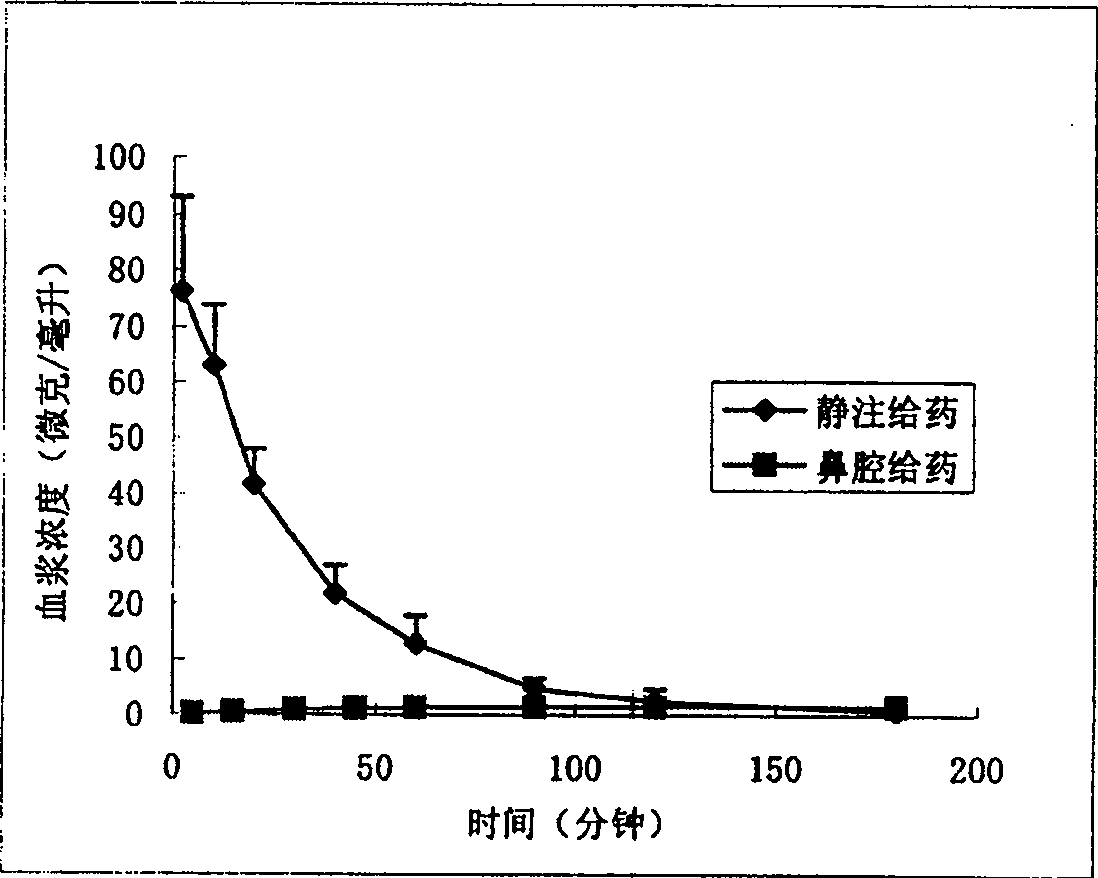
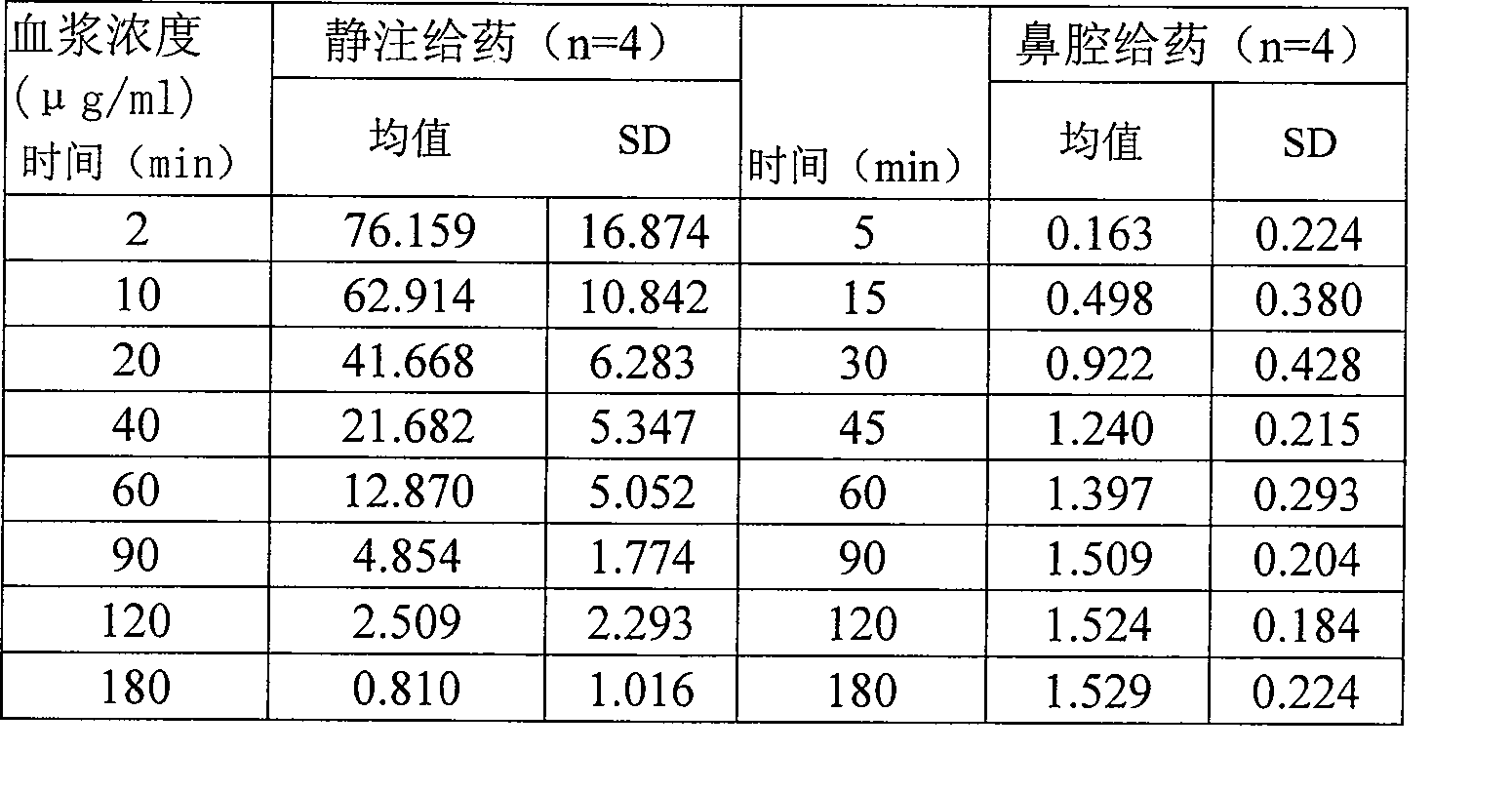
Nose cavity administering formulation of gastrodine
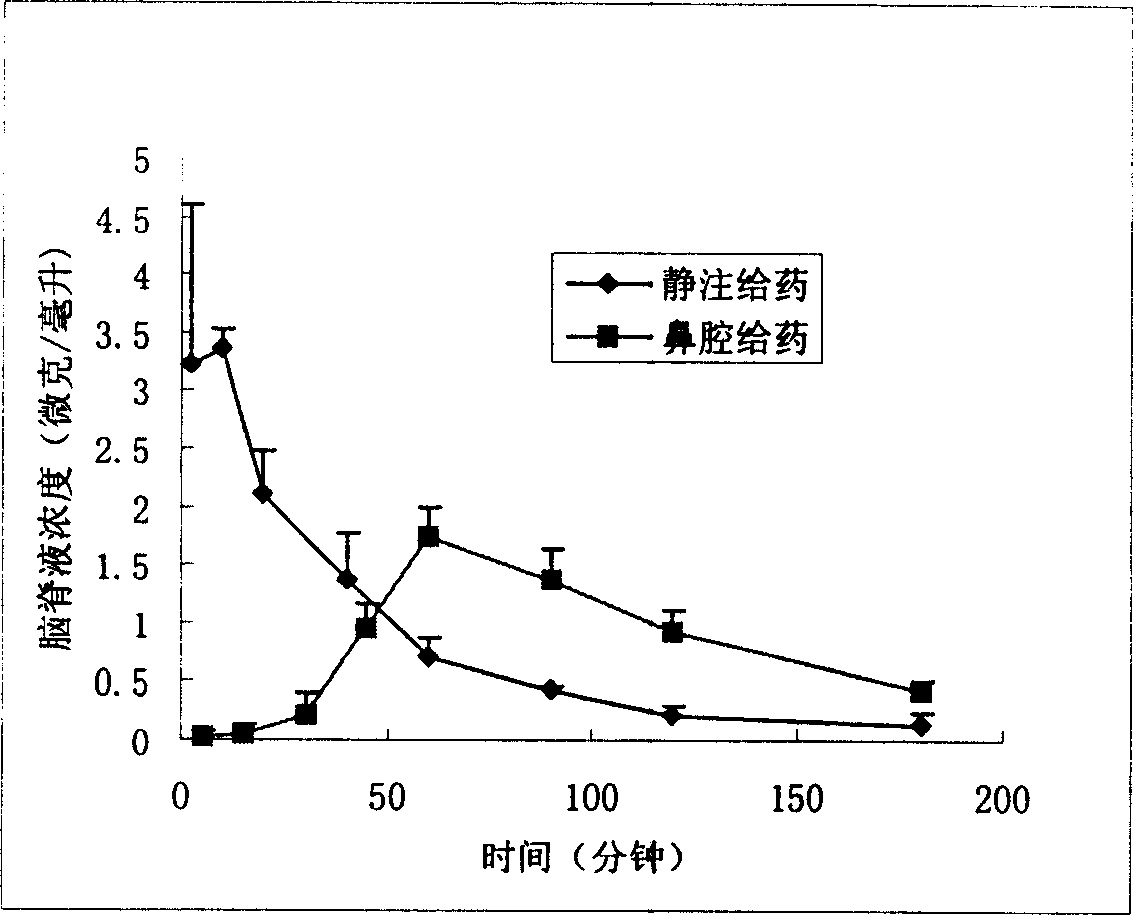
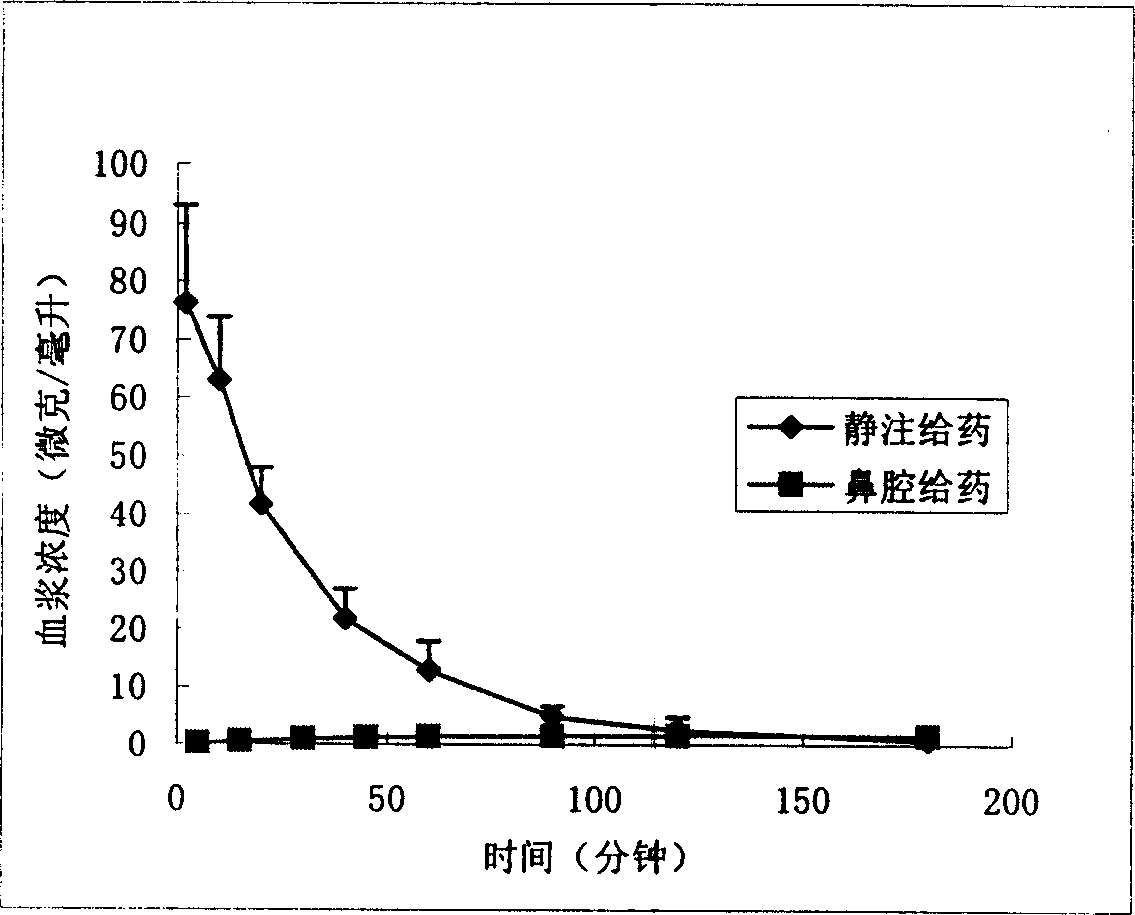
InactiveCN1907290AInto the brain quicklyReduce concentrationOrganic active ingredientsNervous disorderDiseaseNasal cavity
The invention discloses a gastrodine nasal administer drug agent to treat nervous prostration and vascular dementia, which is characterized by the following: adopting gastrodine as main raw material and certain effective drug as auxiliary material; resisting pain; modifying local brain blood flow; targeting brain through gastrodine.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Mesoporous metal organic framework as well as preparation method and application thereof
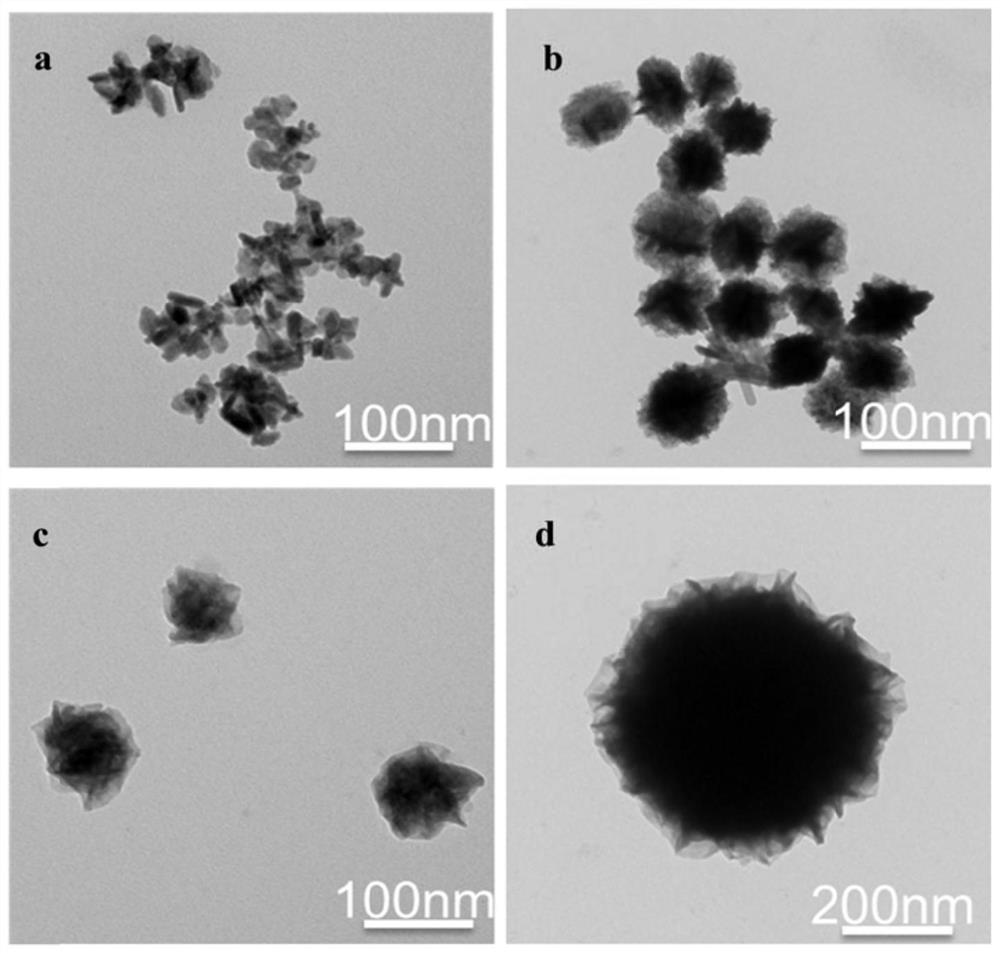
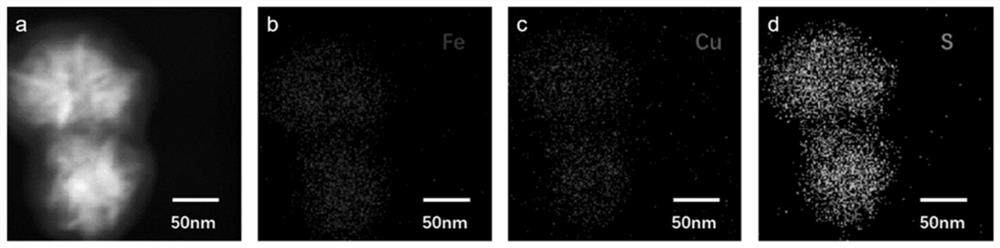
ActiveCN111909384AResponsive to the tumor microenvironmentHigh drug loadingOrganic active ingredientsPhotodynamic therapySide effectMetal-organic framework
The invention provides a mesoporous metal organic framework as well as a preparation method and application thereof, the mesoporous metal organic framework is formed by connecting disulfide bond monomers and metal ions through non-covalent bonds, and the metal ions are Fe 2+ and Cu 2+. The mesoporous metal organic framework disclosed by the invention has the advantages of tumor microenvironment responsiveness, high drug loading capacity, low systemic side effect, good tumor local biodegradability, sufficient drug local release and low systemic side effect, and is expected to promote the further development of tumor ferroptosis and diagnosis and treatment integration.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Vaginal foam preparation of chlorhexidine acetate and its preparing process
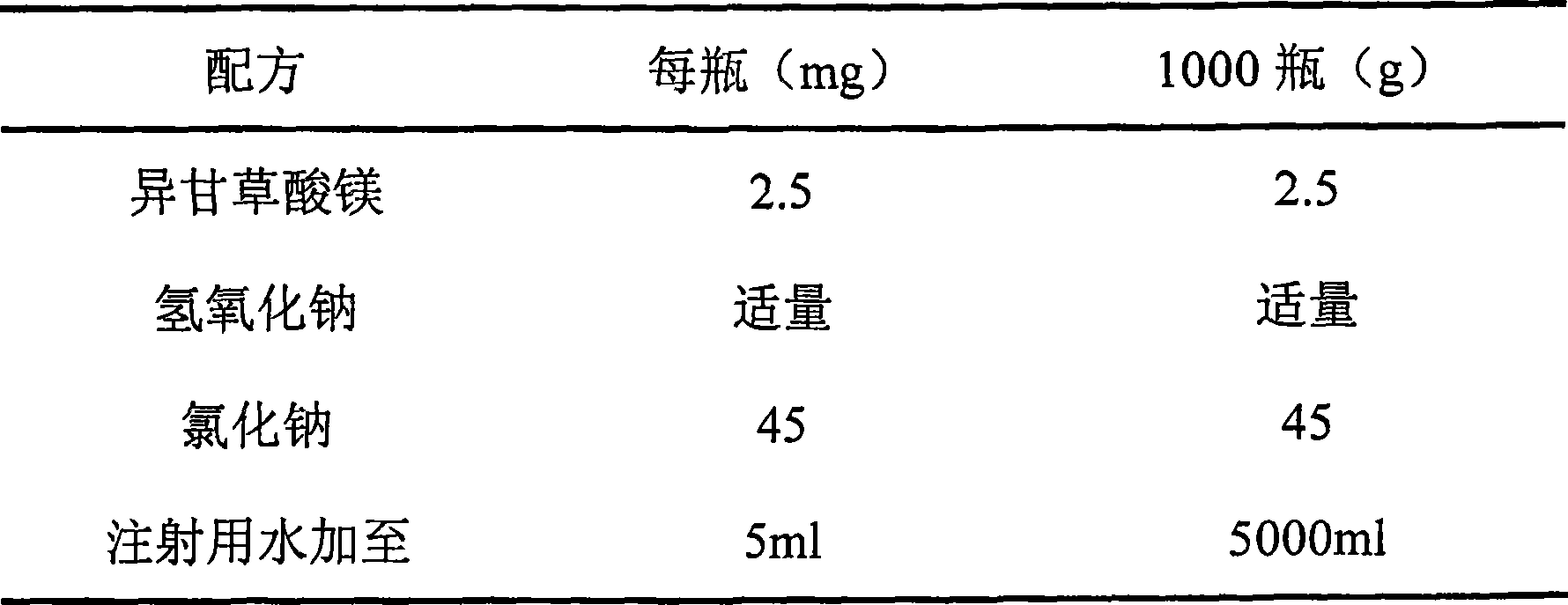
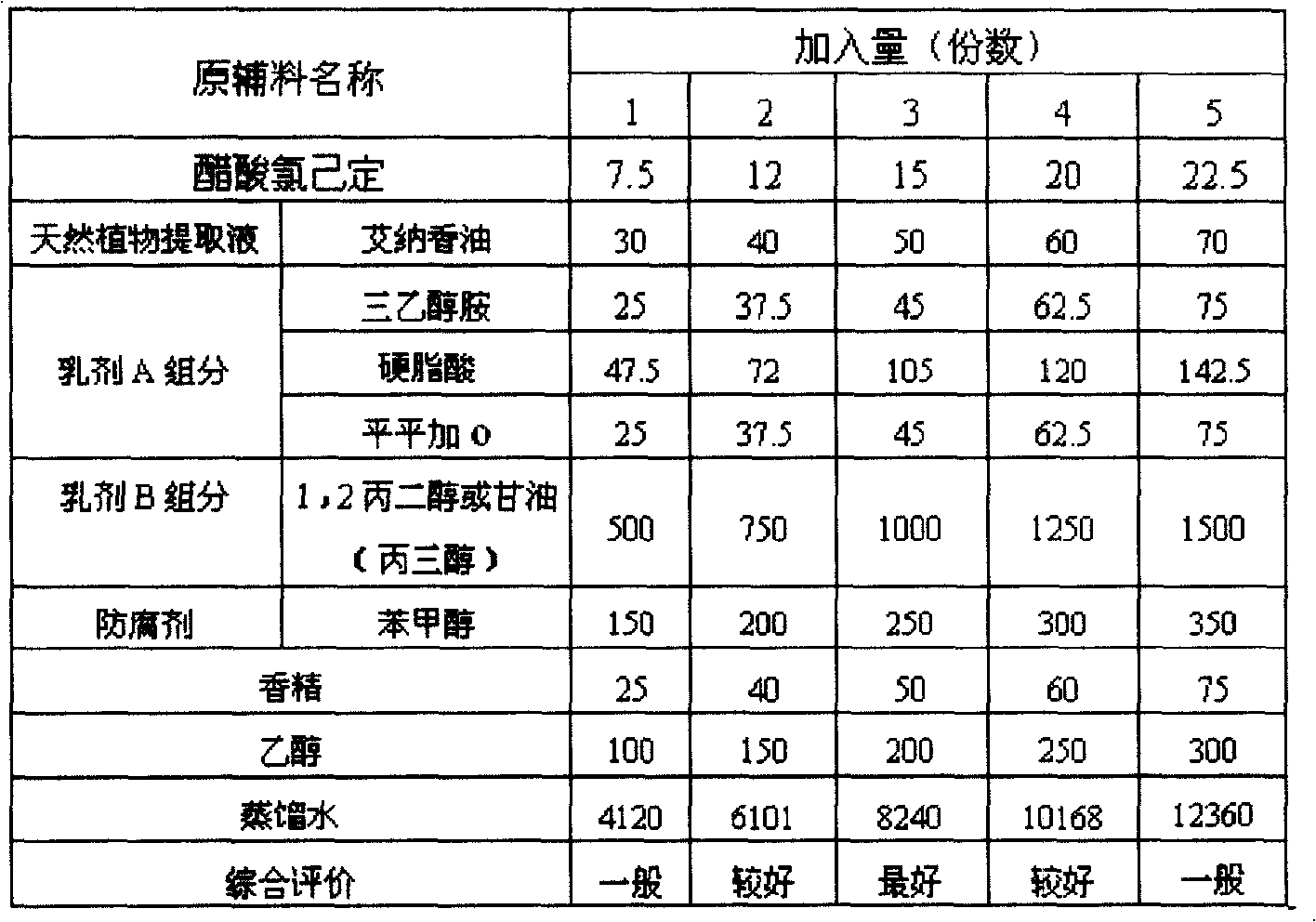
ActiveCN1857243AGood cleaning antibacterial effectSmall systemic side effectsOrganic active ingredientsPharmaceutical delivery mechanismChlorhexidine AcetateBlumea
The present invention discloses vaginal foam preparation of chlorhexidine acetate and its preparation process. The vaginal foam preparation is prepared with chlorhexidine acetate, blumea oil, triethanolamine, stearic acid, peregal O, glycerin, benzyl alcohol, essence, alcohol and water in certain weight proportion. It has excellent cleaning and bacteriostasis effect, and has the advantages of high safety, high stability, less side effect, low dosage, etc. and is suitable for conventional cleaning of vagina.
Owner:贵州宏宇药业有限公司
Injectable temperature-sensitive gel preparation for treating acute pancreatitis
InactiveCN105342984ASmall systemic side effectsSimple preparation processOrganic active ingredientsPeptide/protein ingredientsAcute pancreatitisPolyvinyl alcohol
Critical conditions of acute pancreatitis have high mortality. Trypsinization Theory is one of important pathogeneses of acute pancreatitis. Pancreatin inhibitor drugs, such as gabexate mesylate, ulinastatin and aprotinin, are selectively clinically applied against the mechanism. The routine administration mode of the drugs is intravenous injection, and has certain disadvantages. The invention provides an injectable temperature-sensitive gel preparation for treating acute pancreatitis. The dosage form of the above gel comprises a poloxamer polymer, a polylactide-co-glycolide / polyethylene glycol block copolymer, a polycaprolactam / polyethylene glycol block copolymer, a chitosan / beta-sodium glycerophosphate system, a chitosan / polyvinyl alcohol system or a chitosan-sodium bicarbonate system, or an arbitrary combination thereof. The gel has a reverse gelling characteristic, is a liquid at a low temperature, and converts to a semisolid state at a human body temperature. The invention also provides a preparation method of the injectable temperature-sensitive gel preparation for treating acute pancreatitis.
Owner:GENERAL HOSPITAL OF PLA
Nanoparticles as well as preparation method and application thereof
ActiveCN111603455ANovel mechanismIron-dependentHeavy metal active ingredientsOrganic active ingredientsTherapeutic effectOncology
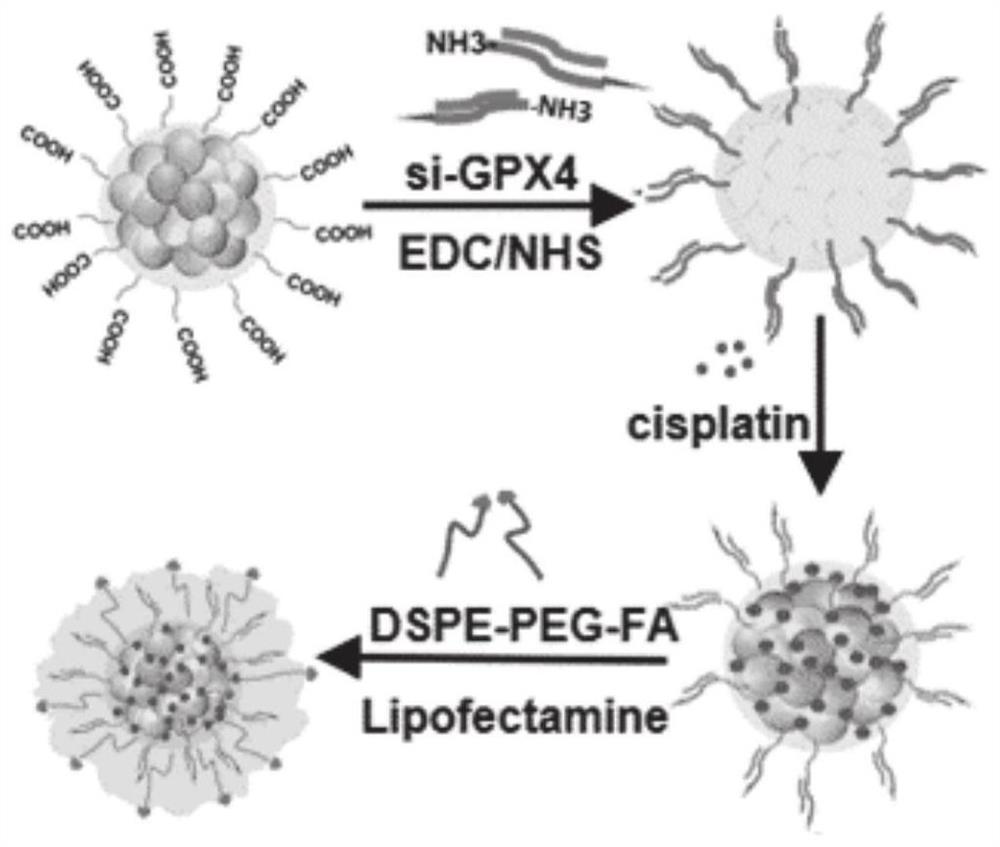
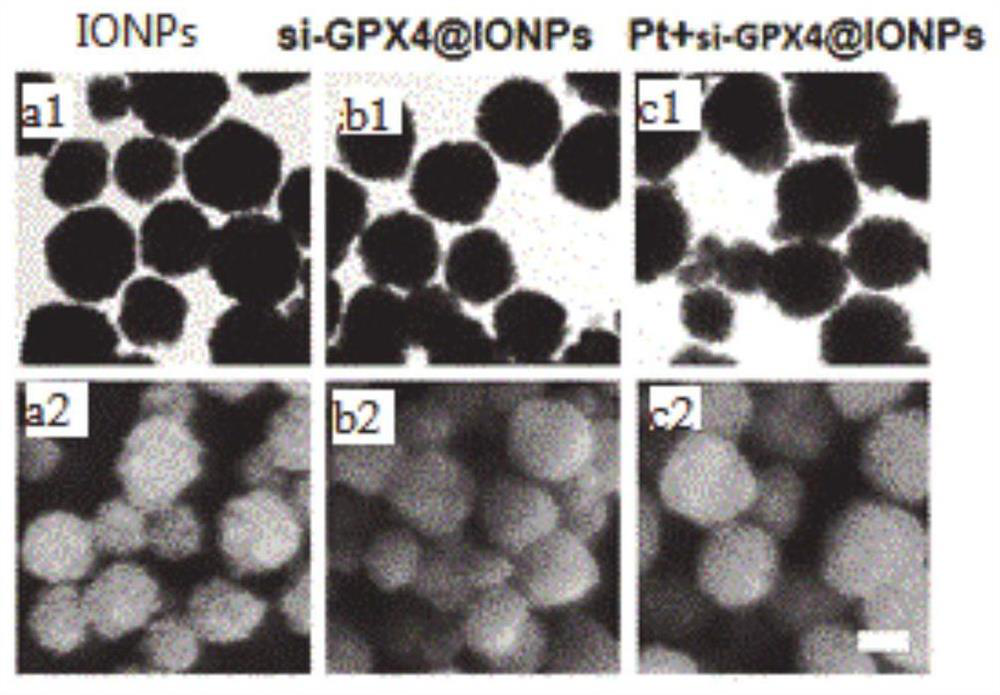
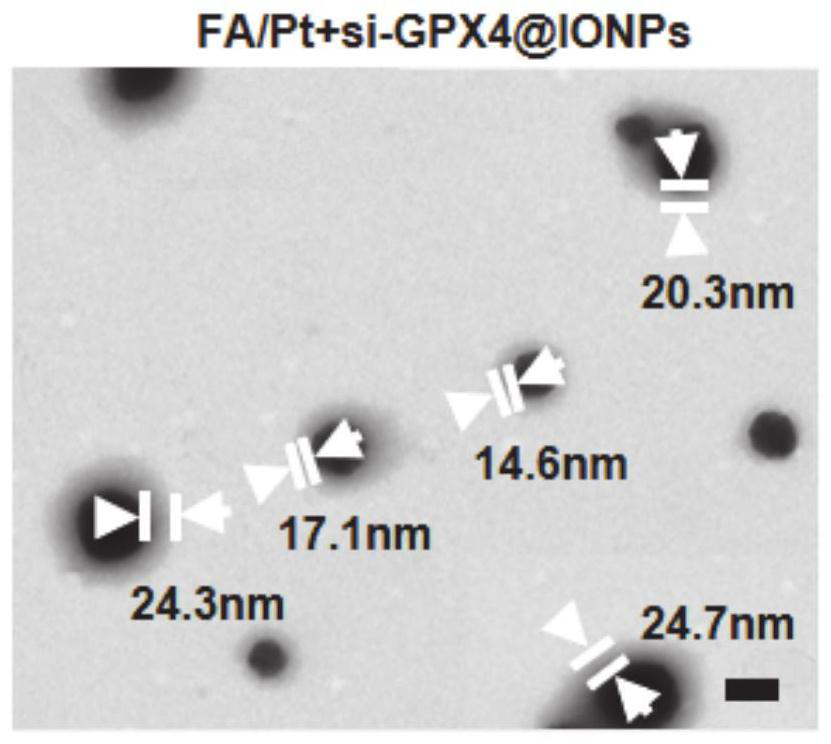
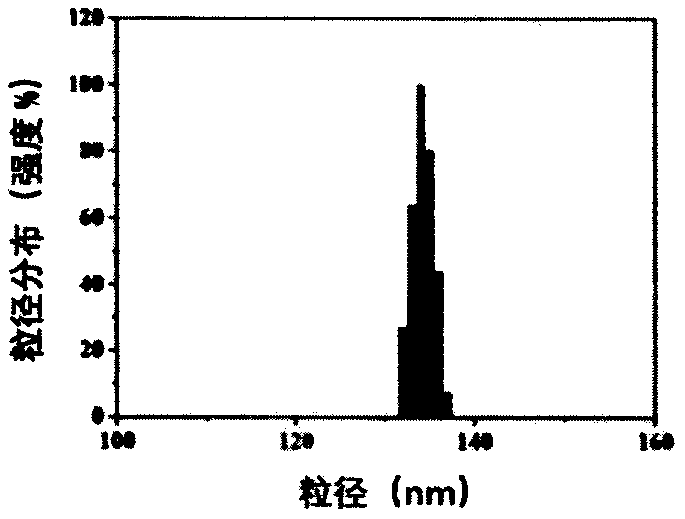
The invention belongs to the technical field of medicines, in particular to Pt+si-GPX4(at)IONPs and FA / Pt+si-GPX4(at)IONPs nano-particles as well as a preparation method and an application thereof. According to the invention, three therapeutic drugs, i.e., cis-platinum, si-GPX4 and IONPs, are combined into a whole; glioma cell death is jointly induced from two aspects of cell apoptosis and ferroptosis; in addition, the FA-modified liposome is used for wrapping three medicines, namely cis-platinum, si-GPX4 and IONPs, the advantages of good targeting property and high biocompatibility are achieved, a very good treatment effect on glioma is achieved, the defects of a traditional single chemotherapy medicine are overcome, and a multi-target combined chemotherapy method for glioma cells is provided.
Owner:SHANDONG UNIV QILU HOSPITAL
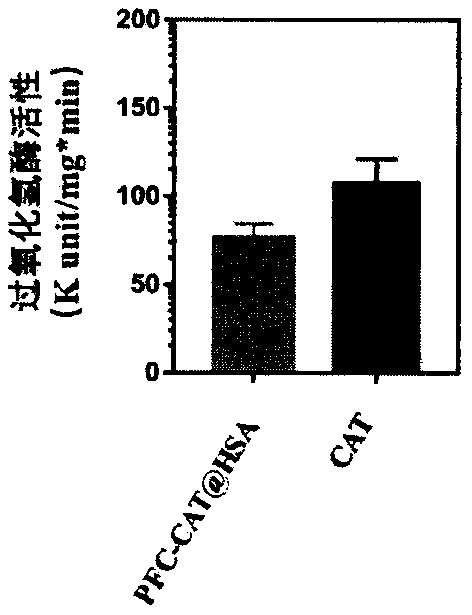
Albumin nanoparticles capable of double-stage oxygen carrying and preparation method and application thereof
ActiveCN109806404AAvoid safety issues such as pulmonary embolismAvoid security issuesPeptide/protein ingredientsRadioactive preparation carriersHigh concentrationConcentration gradient
The invention belongs to the field of radiotherapy sensibilization medicines, and relates to albumin nanoparticles capable of double-stage oxygen carrying and a preparation method and application thereof. The albumin nanoparticles are mainly prepared from albumin, perfluoro-carbon and catalase, the particle size of the albumin nanoparticles is even, the property is stable, the biosecurity is good,and the problem of radiotherapy resisting of solid tumors can be solved. Experimental results show that the nanoparticles can target the tumors and improve tumor hypoxia by means of double-stage oxygen carrying, for specific performance of double-stage oxygen carrying of the nanoparticles, the high-oxygen-carrying perfluoro-carbon directly supplies oxygen (first stage oxygen carrying) to the hypoxic tumors directly through oxygen concentration gradient difference, meanwhile, the catalase conducts catalytic hydrolysis on high-concentration hydrogen peroxide on the tumor portions to generate oxygen for indirect oxygen carrying (second stage oxygen carrying), through synergism of first stage oxygen carrying and second stage oxygen carrying, a tumor hypoxia microenvironment is reversed, and effective sensibilization is conducted on oxygen-dependent tumor radiotherapy.
Owner:南京从一医药科技有限公司
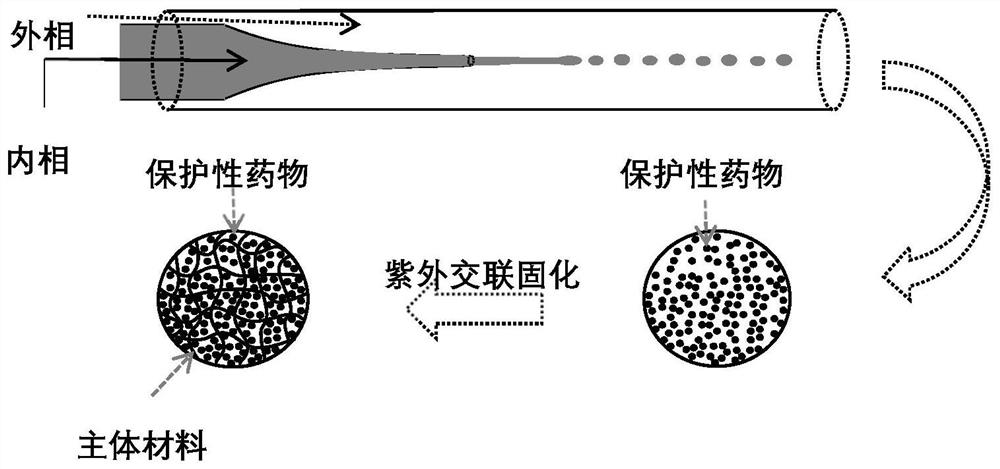
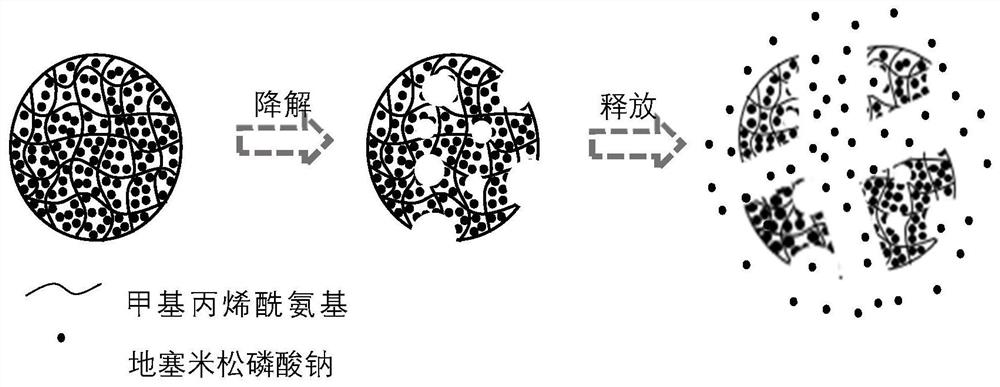
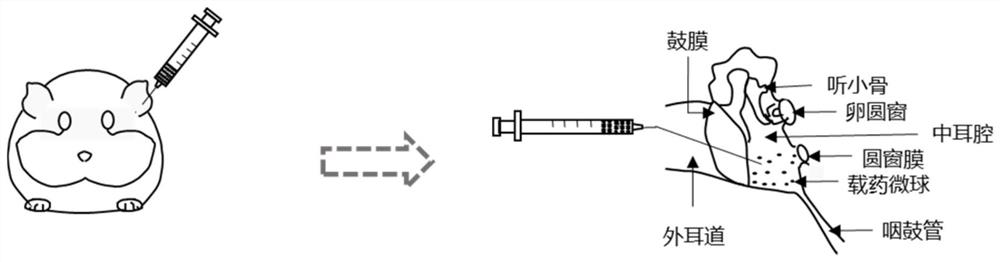
Preparation method of drug microcarrier for acquired deafness based on microfluidic technology
InactiveCN113577030AWide range of optionsThe size is easy to controlOrganic active ingredientsSenses disorderProtective drugsMiddle ear
The invention relates to a preparation method of a drug microcarrier for acquired deafness based on a microfluidic technology, which comprises the following steps of: controlling the generation of internal and external two-phase fluid by utilizing the micro-fluidic technology; the internal and external two-phase fluid generation process comprises the following steps: S1, preparing micron-scale liquid drops capable of being used for tympanic injection by utilizing shearing force and interfacial tension between two-phase fluids which are mutually insoluble, and in-situ entrapping hearing protective drugs and an auxiliary agent for increasing the permeability of a round window membrane; and S2, solidifying the liquid drops through polymerization reaction to form the drug microcarrier. Compared with the prior art, the prepared medicine microcarrier can be used for treating acquired deafness through tympanic injection, the middle ear cavity is filled with the medicine microcarrier through tympanic injection, the medicine is slowly released through natural degradation of the microcarrier and enters the inner ear through the round window membrane, and the effect of treating acquired deafness is achieved; the drug microcarrier has the advantages of small side effect and injury, lasting effect, obvious effect, simple operation and the like.
Owner:FUDAN UNIV
Composition and pharmaceutical dosage form for colonic drug delivery using polysaccharides
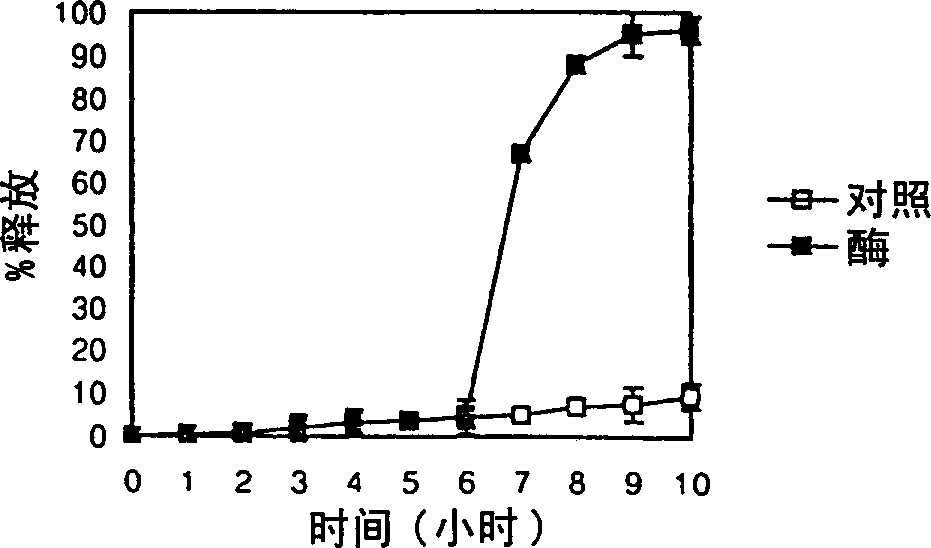
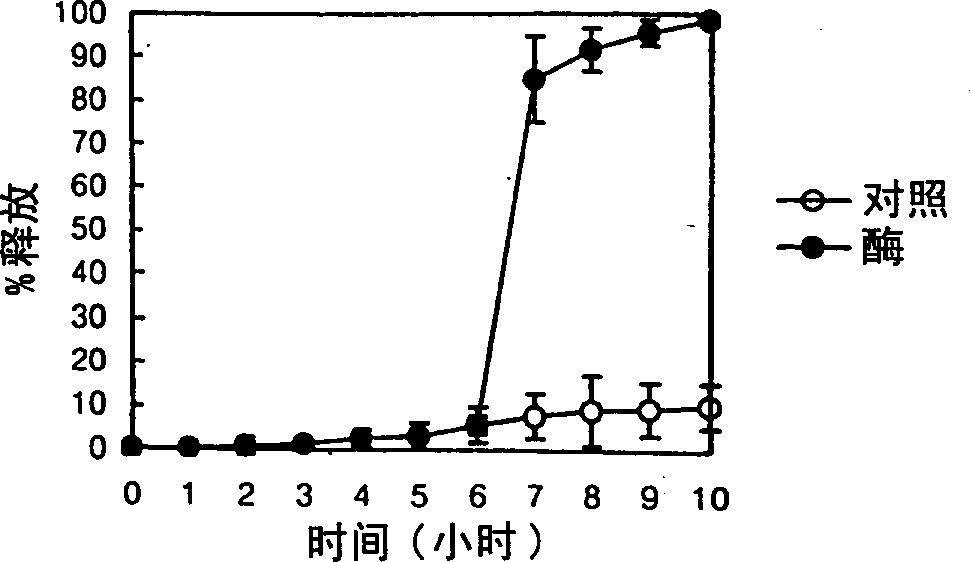
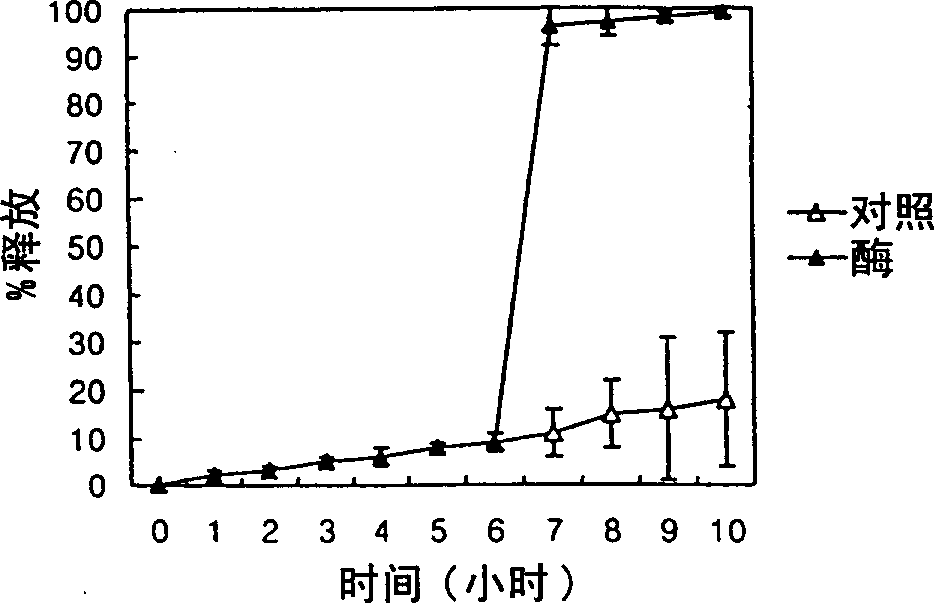
InactiveCN1310630AEfficient releaseSmall systemic side effectsPeptide/protein ingredientsDigestive systemPolysaccharideEnzyme
A colonic drug delivery composition contains a first polysaccharide and a second polysaccharide wherein both polysaccharides are degradable by colonic enzymes and are mixed at a environmental pH of about 7 or above. A colon selective pharmaceutical composition and dosage form for oral delivery of a drug, nutrient, diagnostic reagent, or mixture thereof includes the drug, nutrient, diagnostic reagent, or mixture thereof in contact with the polysaccharide composition. A method of preparing such a colonic drug delivery composition and the colon selective pharmaceutical composition and dosage form are also disclosed.
Owner:SAMYANG BIOPHARMLS CORP
Oral tumor resistant pH sensitive intelligent material and application thereof
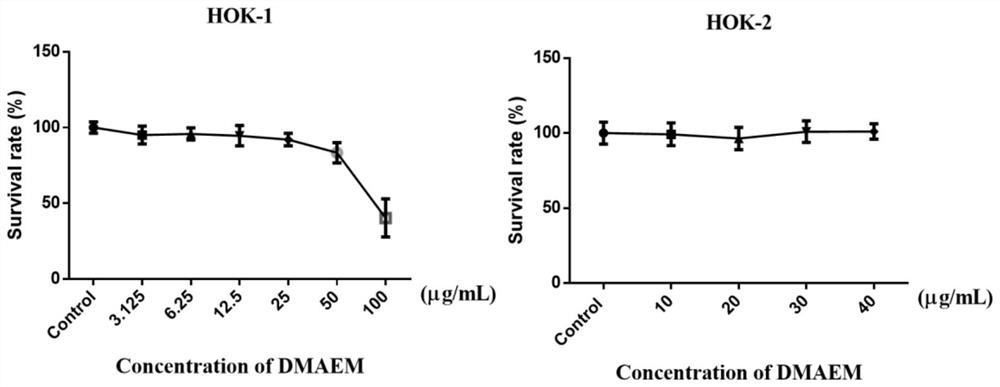
InactiveCN113527115ANo apparent cytotoxicityHigh local effectOrganic compound preparationDigestive systemSquamous CarcinomasOncology
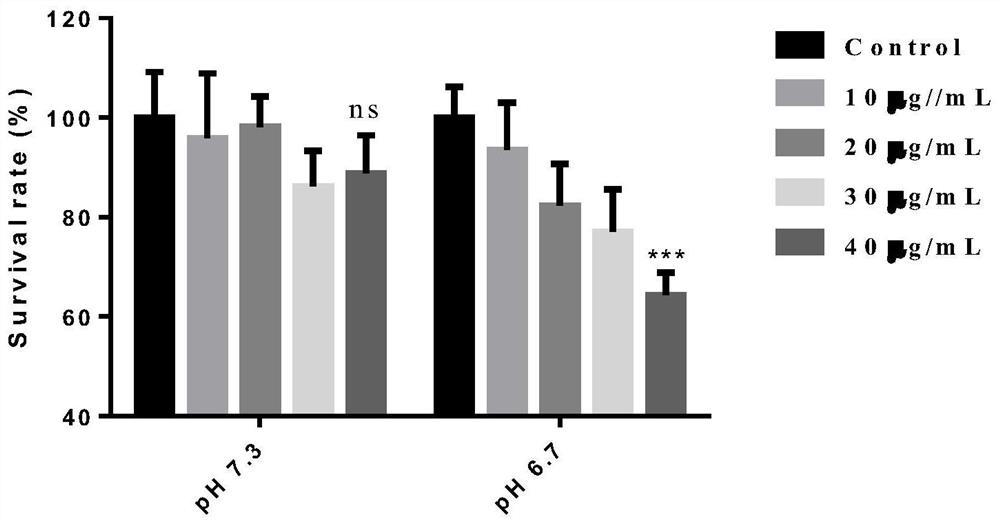
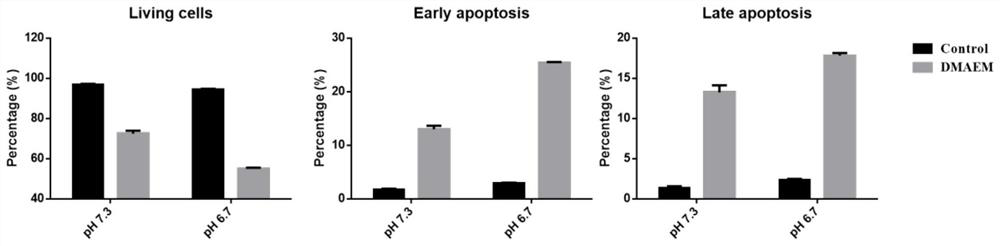
The invention belongs to the technical field of oral tumor resistance, and discloses an oral tumor resistant pH sensitive intelligent material and an application thereof; the oral tumor resistant pH sensitive intelligent material is a tertiary ammonium monomer, and the tertiary ammonium monomer is methylacrylic acid methyl dodecyl amino ethyl ester, namely DMAEM. The invention provides the application of the oral tumor resistant pH sensitive intelligent material in drugs for inhibiting growth of oral squamous cell carcinoma cells, inhibiting tumor growth caused by the oral squamous cell carcinoma cells and preventing or treating the oral squamous cell carcinoma. The oral tumor resistant pH sensitive intelligent material provided by the invention can inhibit proliferation of tumor oral squamous cell carcinoma cells and promote apoptosis of oral squamous cell carcinoma under a biosafety concentration; and in addition, the material has a pH sensitive property, and the proliferation inhibiting and apoptosis promoting effects of the material are enhanced in an acid environment, namely an environment with the pH being 6.7. Animal experiments prove that the material has anti-tumor performance.
Owner:SICHUAN UNIV
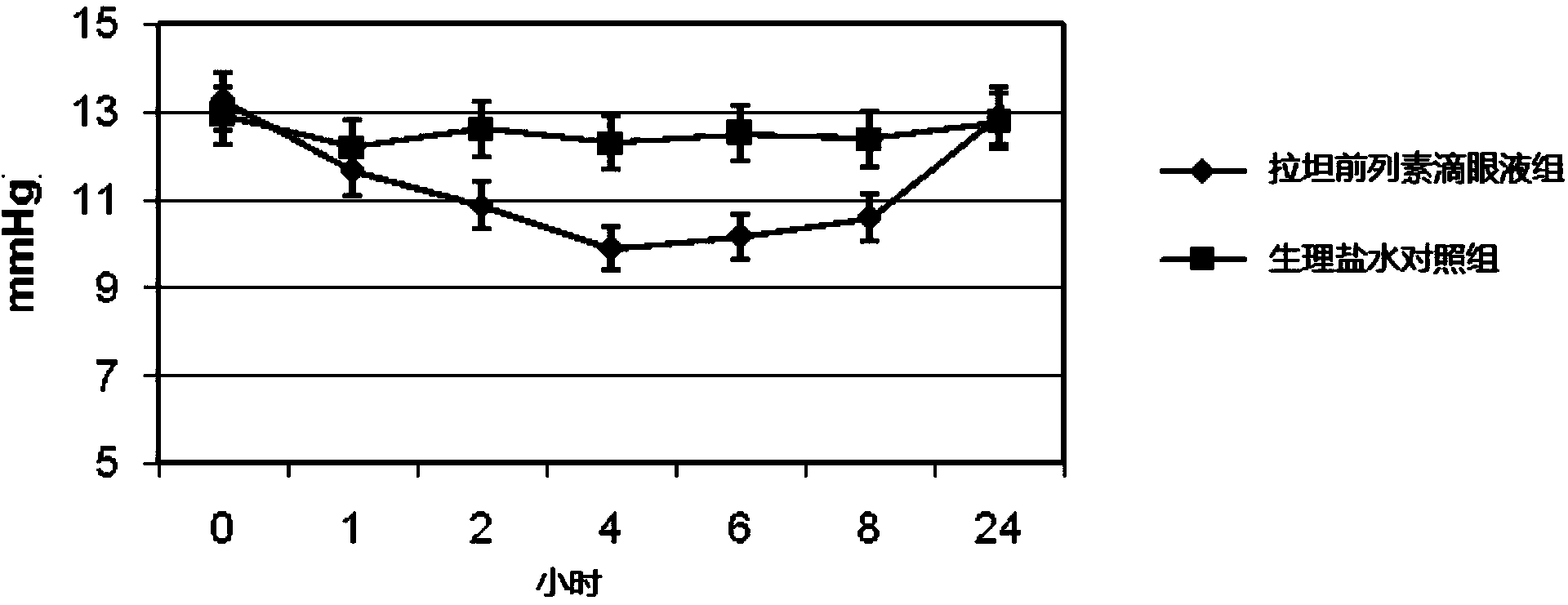
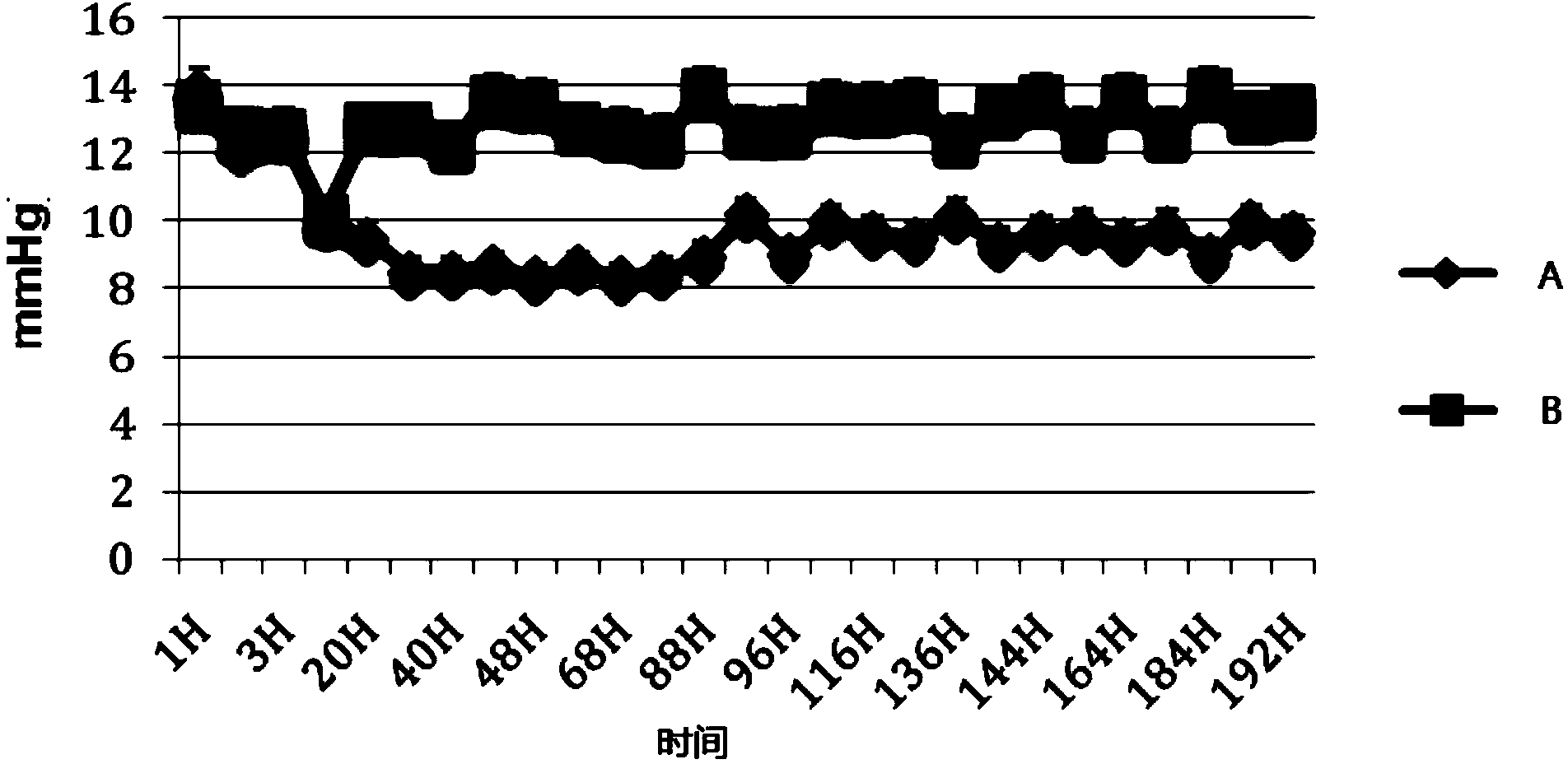
Ganciclovir eye drops and preparation method thereof
InactiveCN102342911AReduce dosageSmall systemic side effectsSenses disorderPharmaceutical delivery mechanismSolventPoloxamer
The invention aims at providing a ganciclovir antiviral new medicament for eye with high efficiency, low toxicity, strong selectivity and good stability, and a preparation method thereof. The medicament provided by the invention comprises 0.1wt% of ganciclovir used as a main medicament, and a cosolvent, an iso-osmotic regulator, a pH regulator, a preservative and water for injection used as auxiliary agents. Compared with the prior art, the medicament has the following advantages that: a boric acid buffer, namely a mixed solution of boric acid and borax is adopted, and the buffer has strong buffering capability and is highly soluble in water; and poloxamer is selected as the cosolvent, and multiple data show that the poloxamer improves the inherent quality of the medicament, increases the preparation stability, reduces the irrigation of anti-inflammation analgesic to eyes, has no hemolysis action as compared with tween-80, has no interference to the bacteriostatic action of the preservative, and has very significant effect on reducing the irritation of medicament to eyes.
Owner:南京恒道医药科技股份有限公司
Chitosan-oligosaccharide-modified self-carrying type carrier-free nasal cavity nano preparation brain-targeted delivery system and preparation method thereof
ActiveCN109730966ANo degradabilityCumulative toxicity NonePowder deliveryNervous disorderNasal cavityFreeze-drying
The invention discloses a chitosan-oligosaccharide-modified self-carrying type carrier-free nasal cavity nano preparation brain-targeted delivery system and a preparation method thereof. The system includes hydrophobic micro-molecule drugs, polyethylene glycol derivatives and chitosan oligosaccharide which have a neuroprotective effect. The invention also provides a preparation method of the nasalcavity nano preparation brain-targeted delivery system. The preparation method includes the steps of 1, preparing nano-particle freeze-dried powder; 2, stirring the freeze-dried powder and chitosan oligosaccharide in isotonic normal saline before use to form a nasal cavity preparation with good membrane permeability. The system is simple in preparation method and can improve the hydrophobicity ofthe micro-molecule drugs, reduce the toxicity and enhance the ne neuroprotective effect. The system is free of carriers, biodegradation problems and accumulation of toxin. The drug carrying rate reaches 25 percent or above, a permeable membrane has good absorption performance after being modified by chitosan oligosaccharide, and the drugs are delivered into the brain with high targeting property.The application modes of the dosage form include nasal dripping, spray and the like, the operation is simple, convenience is provided for patients who have taken the drugs for a long time, and the system has a good application prospect in the aspect of treating nervous system diseases.
Owner:NASAL PHYTO SZ PHARMA TECH CO LTD
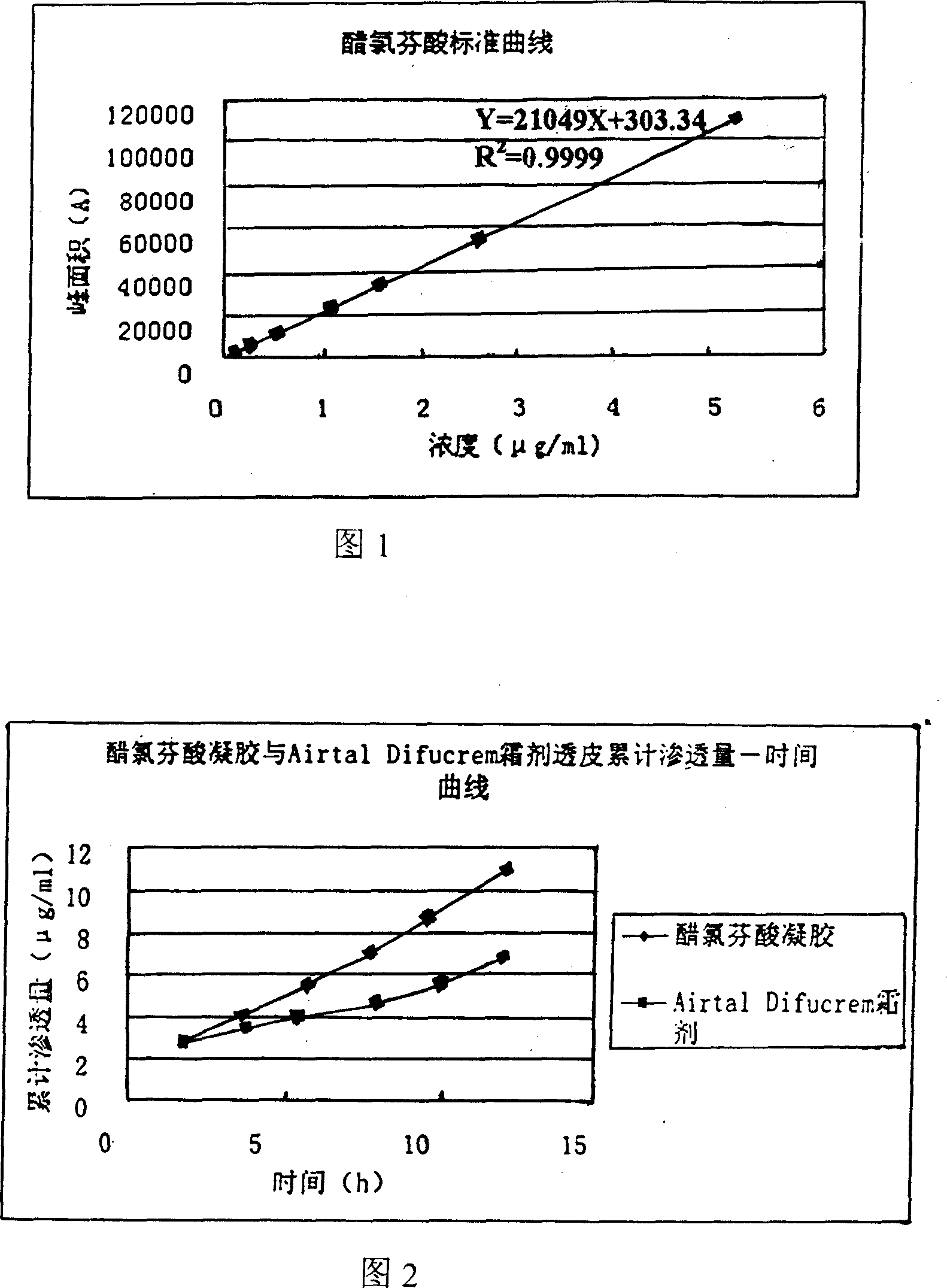
Externally applied aceclofenac gel prepn and its prepn process
InactiveCN101019850ALess irritatingQuality improvementOrganic active ingredientsAntipyreticGel preparationDisease
The present invention discloses one kind of externally applied aceclofenac gel preparation and its preparation process. The externally applied aceclofenac gel preparation is compounded with aceclofenac 0.5-8 wt%, organic solvent 5-50 wt%, bacteriostat 0.001-10 wt%, pH regulator 0.01-2 wt%, carbomer 0.5-8 wt%, antioxidant 0.01-5 wt% and water for the rest. It is prepared through mixing the said ingredients. It is applied to the joint or other disease focus to result in obvious curative effect.
Owner:夏泽宽 +1
Azithromycin eye drops
InactiveCN102579335AAvoid abuseImprove complianceOrganic active ingredientsSenses disorderBacterial ConjunctivitisStaphylococcus aureus
The invention discloses azithromycin eye drops. The azithromycin eye drops are a stable eye preparation which is prepared from azithromycin serving as a main active ingredient and proper auxiliary materials. The long-acting eye drops with mucosa adhesiveness prepared from the latest auxiliary materials at home and abroad have a medicine controlled-release system, the active ingredient stops in eyes for several hours to enhance the antibacterial activity of target tissues, so that the using frequency of the eye drops can be reduced, and the eye drops are convenient to use. The azithromycin eye drops are suitable for treating bacterial conjunctivitis caused by microbial sensitive strains such as G group corynebacterium, haemophilus influenzae, staphylococcus aureus, streptococcus mitis groups and streptococcus pneumoniae.
Owner:GUANGDONG WHOLEWIN TECH
Application of exenatide to preparation of medicines for treating ocular ischemia diseases and improving ocular blood circulation in eye drip way
PendingCN111166870ABroaden the field of studyReduce fearSenses disorderPeptide/protein ingredientsRetinal capillaryBlood circulating
The invention relates to the technical field of medicines, in particular to application of exenatide to preparation of medicines for treating ocular ischemia diseases and improving ocular blood circulation in an eye drip way. The exenatide can be used for treating the ocular ischemia diseases such as onset of acute glaucoma and improving the ocular blood circulation . According to the applicationdisclosed by the invention, through building an acute ischemia reperfusion model and simulating damage of the onset of the acute glaucoma, findings prove that an eye drop preparation of Exendin-4 canimprove a retinal vascular endothelial function, dilating retinal capillary and recovering fundus blood circulation, so that the damage to the fundus blood supply caused by the ocular ischemia diseases such as acute glaucoma can be effectively alleviated, and simultaneously, the findings prove that a new medicine dosage form (eye drop preparation) can achieve an effective local action under the premise of avoiding systemic action. The exenatide disclosed by the invention can be used for treating deficiency of the fundus blood supply such as the acute glaucoma, retinopathy of prematurity and retinal vascular occlusion diseases, and thus, the visual function is saved.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
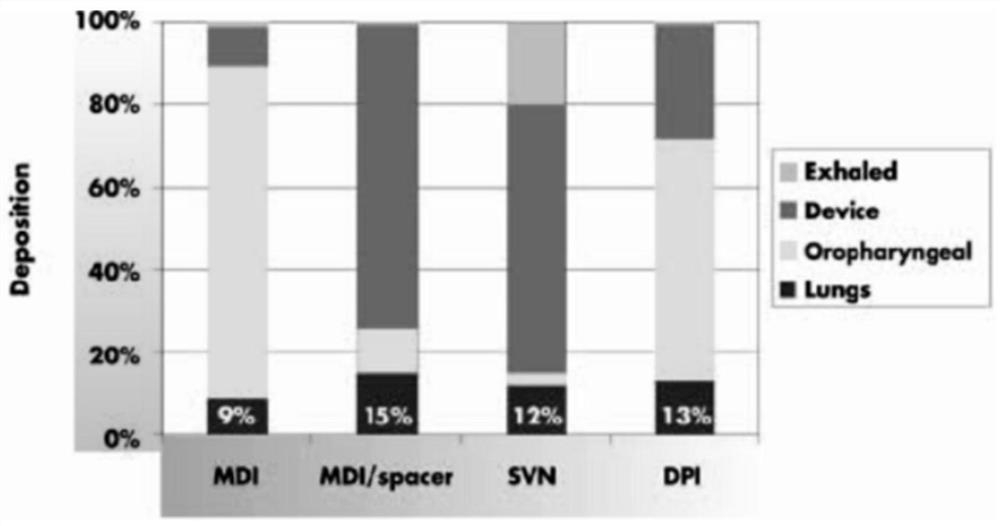
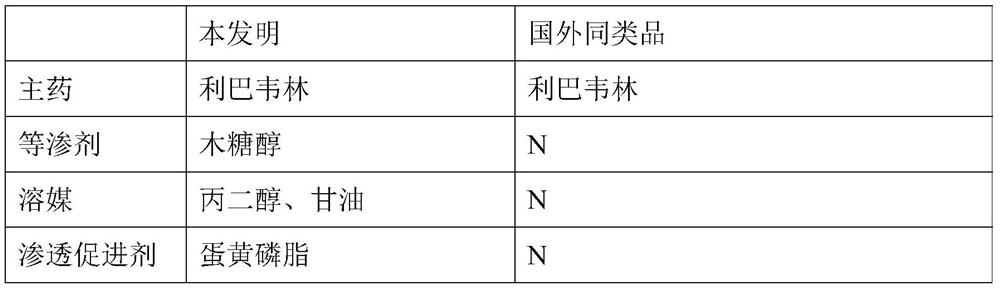
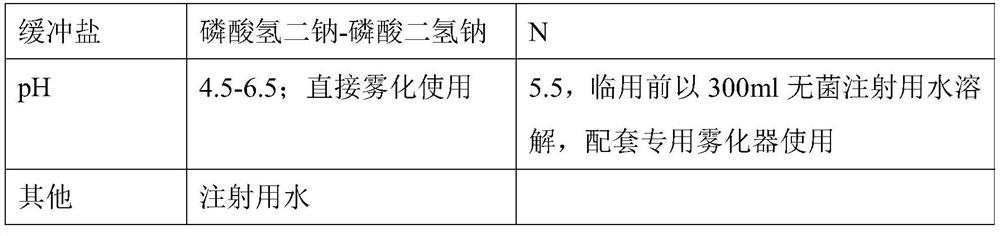
Solution used for Ribavirin aerosol inhalation, and preparation method of solution
ActiveCN113491676AGuaranteed stabilityIncrease concentrationOrganic active ingredientsDispersion deliveryCyclodextrinPhospholipid
The invention discloses a solution used for Ribavirin aerosol inhalation, and a preparation method of the solution. Each ml of the solution used for the Ribavirin aerosol inhalation comprises: 1) 0.005-5 g of Ribavirin or pharmaceutically acceptable salts thereof, 2) 0.1-0.5 mg of isotonic agent, 3) 0.2-0.8 mg of buffer salt, 4) 0.3-4.0 mg of penetration enhancer, 5) 0-50 mg of cyclodextrin, 6) 0.1-1.0 mg of solvent, and the balance of water for injection. According to the invention, phospholipid is innovatively used as the penetration enhancer, so that the solubility of the medicine in a liquid state is improved, and the penetration enhancer improves a delivery ratio in the respiratory tract, especially in pulmonary alveoli, and accelerates absorption. The medicine preparation provides a treatment medicine and a treatment scheme which are lacked in the prior art and have the advantages of an accurate medicinal dosage, high and stable medicine quality and safe, simple and convenient clinical application.
Owner:SHIJIAZHUANG DISCOVERY MEDICINE TECH CO LTD
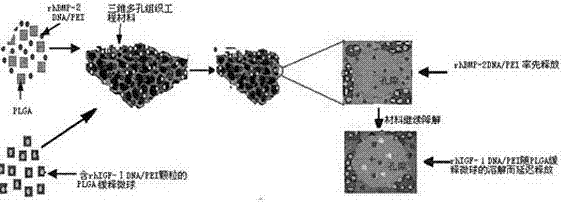
Preparation method of double-gene time sequence sustained-release tissue engineering scaffold material
ActiveCN107412859APromote regenerationPromotes defect repairGene therapyProsthesisChemistrySide effect
The invention discloses a preparation method of a double-gene time sequence sustained-release tissue engineering scaffold material. The preparation method comprises the following steps: firstly, encapsulating plasmid containing IGF-I by virtue of a sustained-release material which is relatively high in molecular weight, so that sustained-release microspheres are prepared; and preparing the three-dimensional porous tissue engineering material from the microspheres, PLGA which is relatively low in molecular weight and plasmid containing BMP-2 by virtue of a supercritical CO2 and particulate leaching method. As the material is implanted into a body, a main body material, which is relatively low in molecular weight, around the BMP-2 plasmid which is not encapsulated in sustained-release microspheres is degraded firstly, and the BMP-2 plasmid is released firstly; while the IGF-I plasmid, which is encapsulated in the material, which is relatively high in molecular weight, is delayed in release, so that an effect that two target genes are released in a certain order. According to the material, cell factors can be subjected to time sequence expression in a mode of simulating a physiological process of tissue repair, so that the material is more conducive to tissue regeneration and systemic side effects are reduced; a gene product can achieve local continuous release; and a local therapeutic effect can be enhanced to the greatest extent.
Owner:温州医科大学附属口腔医院
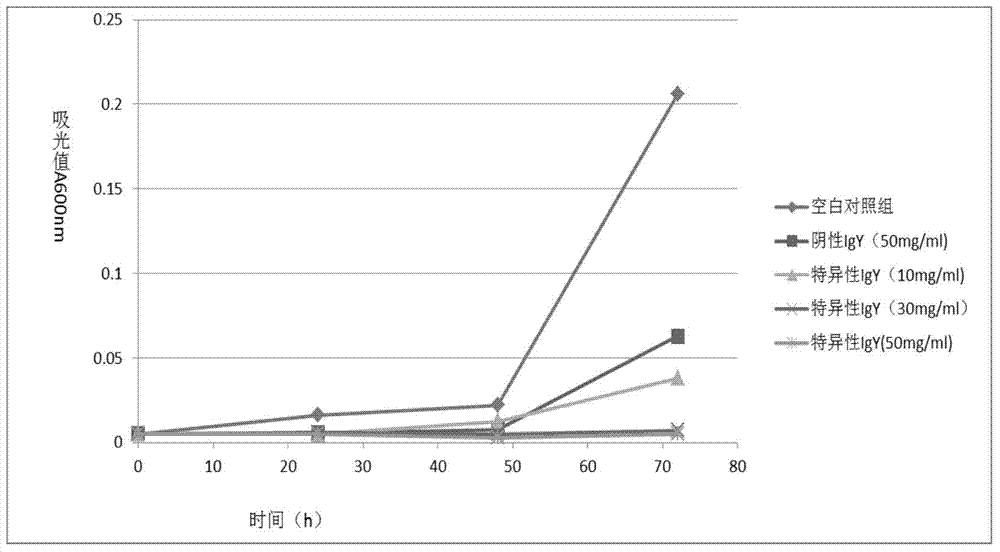
Preparation for specific aspergillus fumigatus infection resisting chicken egg yolk antibody and application
InactiveCN104844709ASimple preparation processLow costSenses disorderEgg immunoglobulinsSnow moldAnimal science
The invention relates to the technical field of immunity, in particular to a specific aspergillus fumigatus infection resisting chicken egg yolk antibody and application thereof. The specific aspergillus fumigatus infection resisting chicken egg yolk antibody is prepared by the following steps: 1, preparing an aspergillus fumigatus antigen; 2, performing immune injection on laying hens; 3, preparing specific chicken egg yolk antibody freeze-dried powder by utilizing an ammonium sulfate salting-out method. The valence of the prepared specific aspergillus fumigatus infection resisting chicken egg yolk antibody is higher than 1:10,000; the prepared specific aspergillus fumigatus infection resisting chicken egg yolk antibody is a biological agent, the side effects generated to the human body are small, the irritation to human body conjunctivas, corneas, mucosa, skin and the like is small, no adverse reaction exists when the antibody is externally used or orally taken, and the physicochemical property is stable; the antibody can be saved for a long time and is particularly suitable for being locally applied to treating aspergillus fumigatus infection of ocular surface, oral cavity, skin and the like.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV +1
Phacolysin eye in-situ gel
InactiveCN101336919AExtended stayReduce absorptionOrganic active ingredientsSenses disorderAdjuvantSide effect
The invention discloses a phacoline eye gel and a preparation method thereof. The phacoline eye gel contains the following components (per 1,000 mL) phacoline 50-150 mg, antiseptic 0.05-1 g, osmotic pressure regulator 1.0-7.5 g, gel matrix 30-150 g, and acid / base buffer and water for injection in balancing amount. The inventive phacoline eye gel optimizes adjuvants and improves production process, so as to enrich the phacoline dosage forms, remarkably prolong the retention time of the phacoline in eyes, improve the therapeutic effect, reduce eye drop frequency, and prevent the occurrence of cataract and glaucoma. In addition, the eye gel has reduced fluidity and can reduce the drug absorption in lacrimal passage and nasopharyngeal mucosa, so as reduce systematic side effects.
Owner:肖正连
Vaginal foam preparation of chlorhexidine acetate and its preparing process
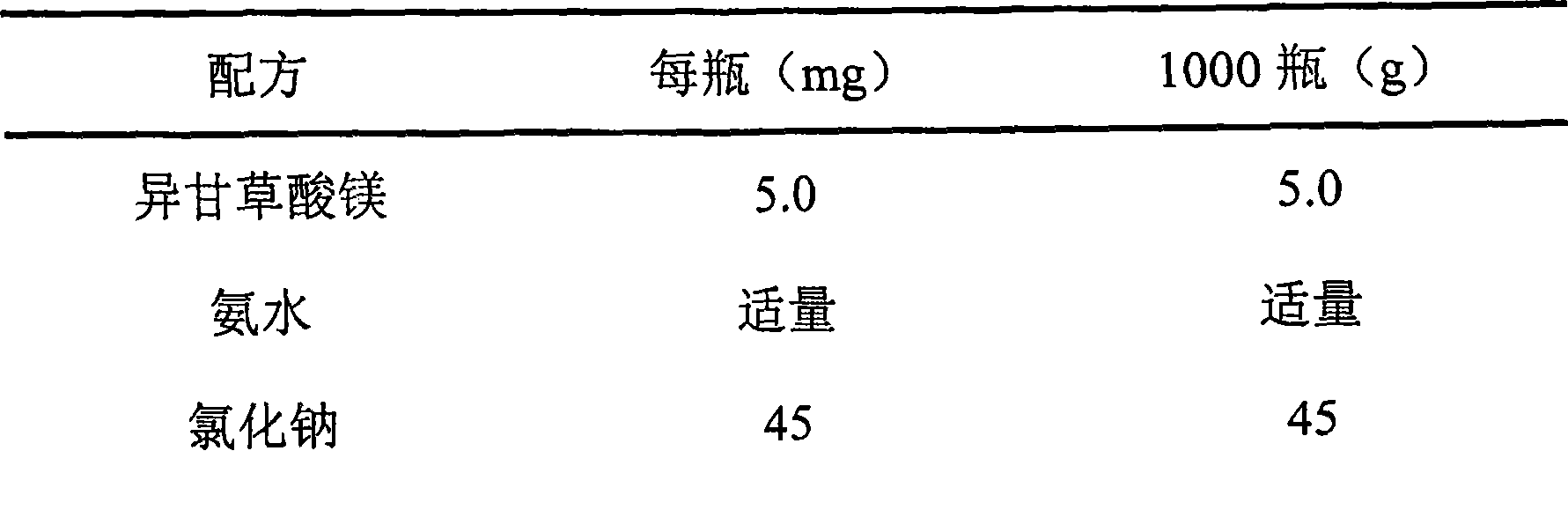
ActiveCN100427079CGood cleaning antibacterial effectSmall systemic side effectsOrganic active ingredientsPharmaceutical delivery mechanismChlorhexidine AcetateBlumea
The present invention discloses vaginal foam preparation of chlorhexidine acetate and its preparation process. The vaginal foam preparation is prepared with chlorhexidine acetate, blumea oil, triethanolamine, stearic acid, peregal O, glycerin, benzyl alcohol, essence, alcohol and water in certain weight proportion. It has excellent cleaning and bacteriostasis effect, and has the advantages of high safety, high stability, less side effect, low dosage, etc. and is suitable for conventional cleaning of vagina.
Owner:贵州宏宇药业有限公司
Alprostadil nano granule formulation and preparation thereof
InactiveCN101322712BReduce chance of regroupingOvercome stabilityPowder deliveryOrganic active ingredientsPulmonary vasculatureDisease
The invention relates to an alprostadil nanoparticle preparation and a preparation method thereof. The preparation is essentially used for transdermal administration and used for treating diseases such as diabetic ulcer, ischemia of extremity end, burn and the like, and belongs to the technical field of medicine. The preparation consists of the raw materials according to the following weight ratio: alprostadil: lipid component: emulsifier: excipient matrix: water is 0.01-0.1:0.5-10:0.5-5:550-950:5-95. The alprostadil nanoparticle preparation has the advantages of overcoming the chemical and physiological instability of the alprostadil, strengthening in vitro stability, reducing degradation of the alprostadil caused by inactivation effect of in vivo pulmonary circulation, enhancing concentration of the preparation on the local affected parts, being more easy to aggregate at the affected part, sustained release and long effect, and reducing irritation of the medicine.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Nose cavity administering formulation of gastrodine
InactiveCN100542540CInto the brain quicklyReduce concentrationOrganic active ingredientsNervous disorderNasal cavityDisease
The invention discloses a gastrodine nasal administer drug agent to treat nervous prostration and vascular dementia, which is characterized by the following: adopting gastrodine as main raw material and certain effective drug as auxiliary material; resisting pain; modifying local brain blood flow; targeting brain through gastrodine.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
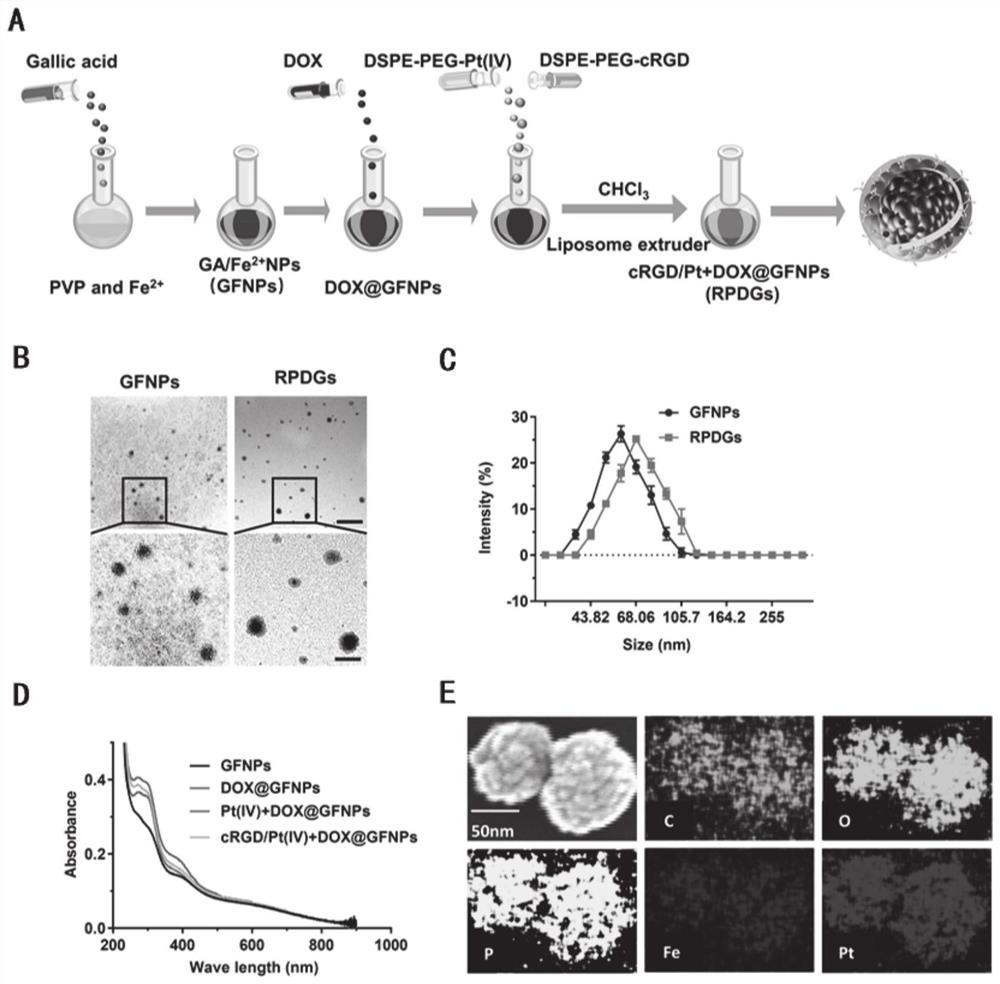
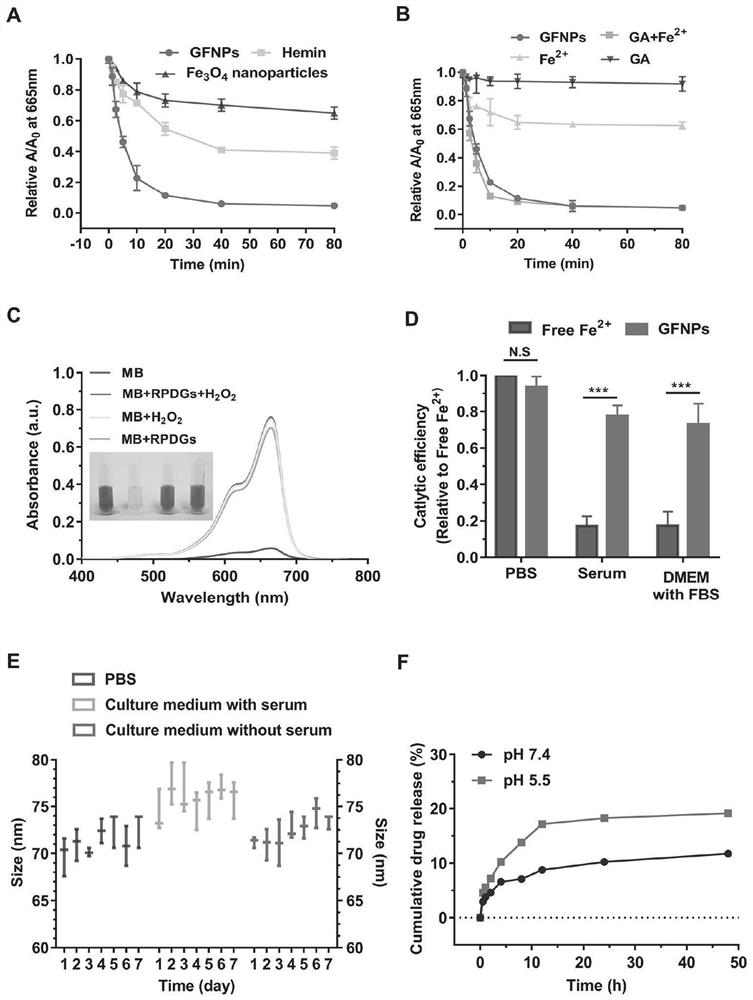
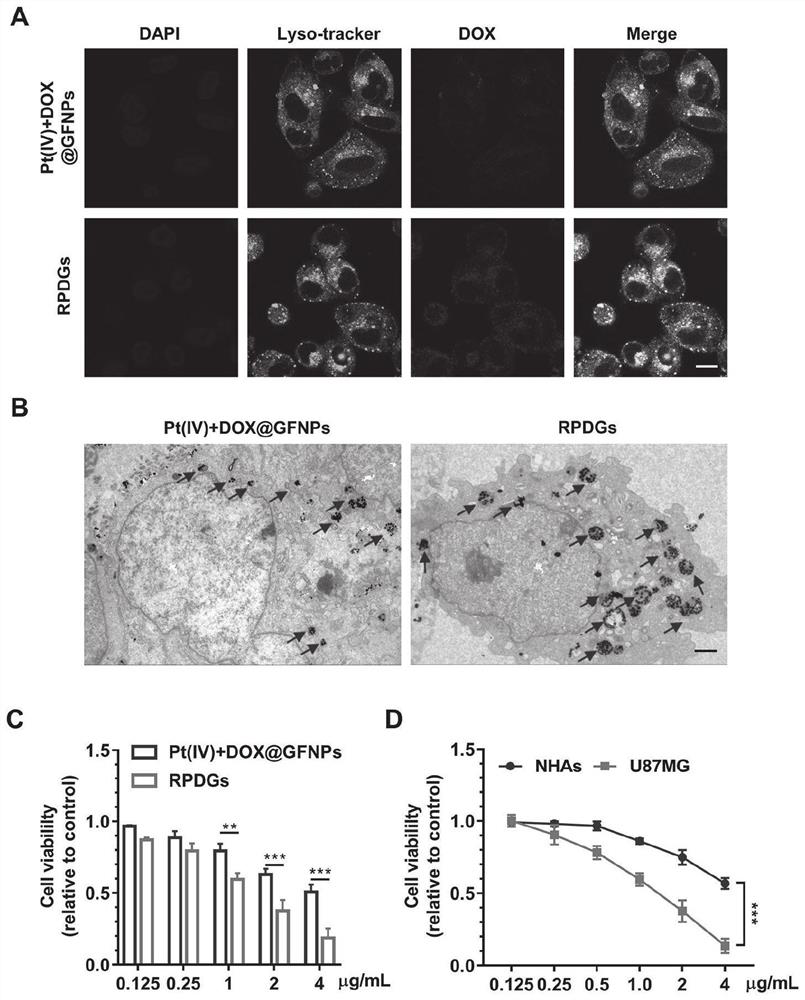
Platinum (ⅳ) and crgd-modified ga/fe nanoparticles loaded with doxorubicin and its method for targeted treatment of tumors
ActiveCN113521098BNovel mechanismIron-dependentOrganic active ingredientsMaterial nanotechnologyTumor therapyInducer Cells
Owner:SHANDONG UNIV QILU HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com