Externally applied aceclofenac gel prepn and its prepn process
A technology of aceclofenac and gel, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve research problems and achieve safe and convenient use, no toxic and side effects, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
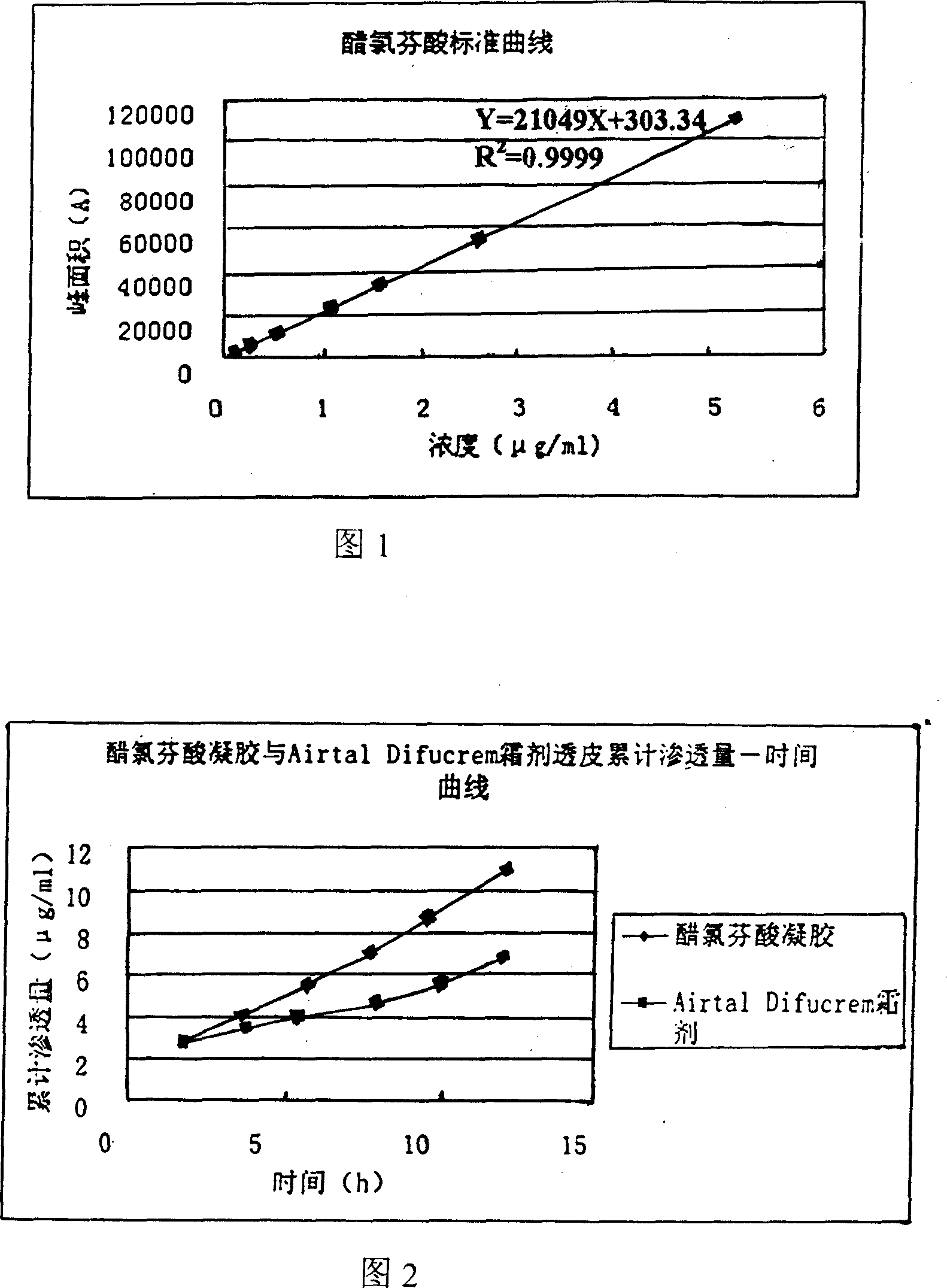
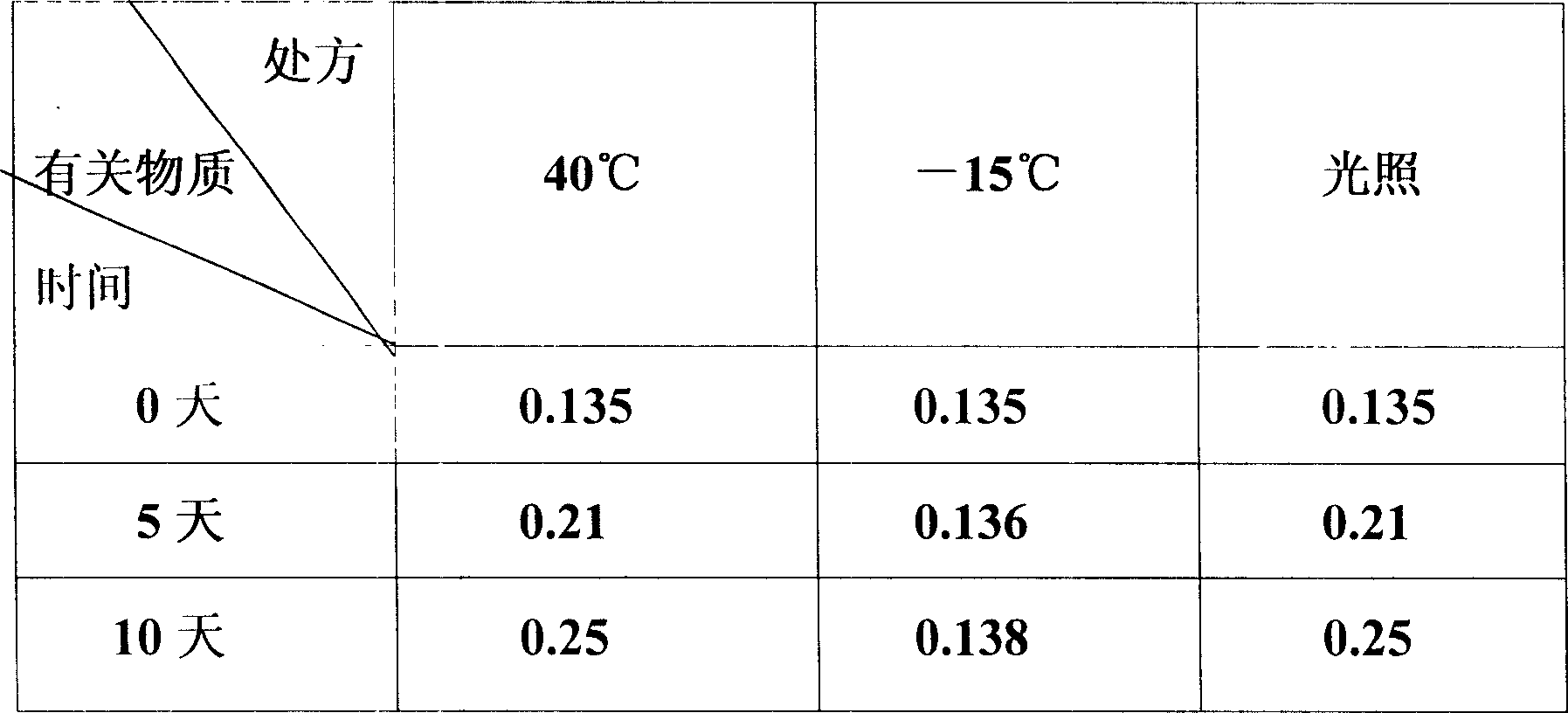
Image
Examples
Embodiment Construction
[0033] The present invention provides following embodiment, the present invention is described further:
[0034] Based on 100g of medicament, the weight ratio of the following components is:
[0035] 1. Aceclofenac 0.5g, propylene glycol 5g, ethylparaben 10g, sodium carbonate 0.01g, carbomer 940 0.5g and thioglycerol 5g, the balance is water.
[0036] 2. 7g of aceclofenac, 10g of propylene glycol, 0.001g of ethylparaben, 0.03g of sodium carbonate, 0.5g of carbomer 940 and 0.01g of thioglycerol, and the balance is water.
[0037] 3. Aceclofenac 8g propylene glycol 20g, ethylparaben 0.2g, sodium carbonate 1.5g, carbomer 940 2g and vitamin Clg, and the balance is water.
[0038] 4. Aceclofenac 3g, propylene glycol 25g, benzalkonium chloride 1.5g, sodium carbonate 0.2g, carbomer 940 1g, thioglycerol 3g, and the balance is water.
[0039] 5. Aceclofenac 4g, ethanol 30g, thimerosal 2g, sodium carbonate 2g, carbomer 9417g and thioacetic acid 2g, the balance is water.
[0040] 6. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com