Platinum (ⅳ) and crgd-modified ga/fe nanoparticles loaded with doxorubicin and its method for targeted treatment of tumors
A nanoparticle and doxorubicin technology, which is applied in the field of biomedicine and tumor treatment, can solve the problems of poor curative effect, limited surgical resection range, and easy drug resistance, so as to achieve exact inhibitory effect, reduce drug resistance, Good targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0081] Materials and Methods
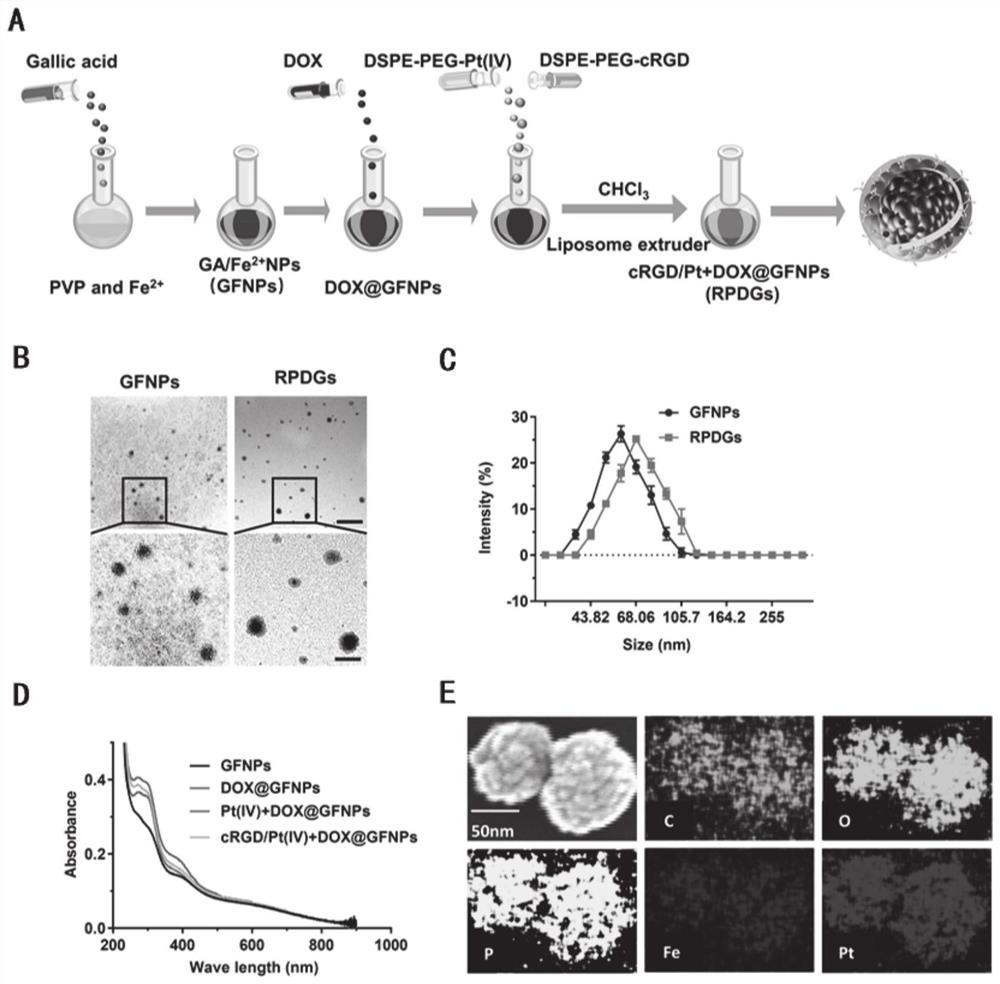
[0082] 1. Preparation of DSPE-PEG(2000)Pt(IV) and DSPE-PEG(2000)-cRGD: the cis, cis and trans [PtIV(NH 3 ) 2 Cl 2 (O 2 CCH 2 CH 2 CO 2 h) 2 ] (9.6mg, 18.0μmol), dicyclohexylcarbodiimide (3.7mg, 18.0μmol) and 4-(dimethylamino)pyridine (1.0mg, 7.2μmol) were dissolved in DMSO (130μL), after 10min the The solution containing the active Pt(IV) complex was added to the prepared DSPE-PEG(2000)-NH 2 (10mg, 3.6μmol, 170μL) DMSO solution, the resulting mixture was continuously stirred and reacted at room temperature for 72 hours, and the reaction product was centrifuged with water, and the supernatant was dialyzed and purified on an ultrafiltration filter with a molecular weight of 2000 to obtain DSPE- PEG(2000)Pt(IV). In the same way, cRGD was coupled with PEGylated phospholipids, and DSPE-PEG(2000)-cRGD was obtained by dialysis and purification.
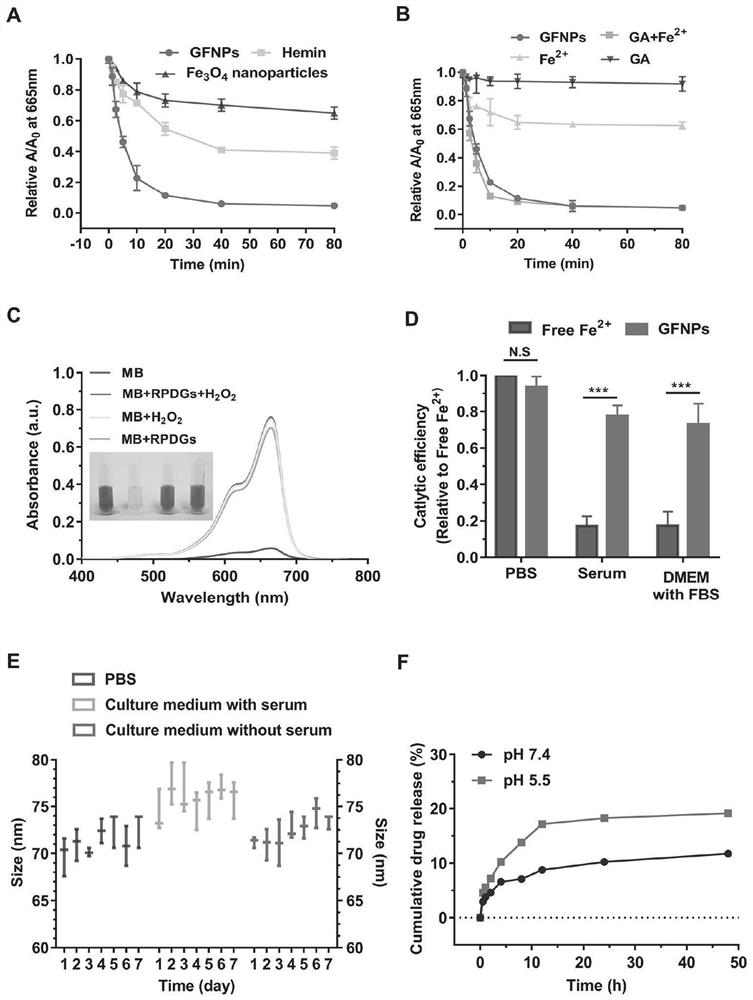
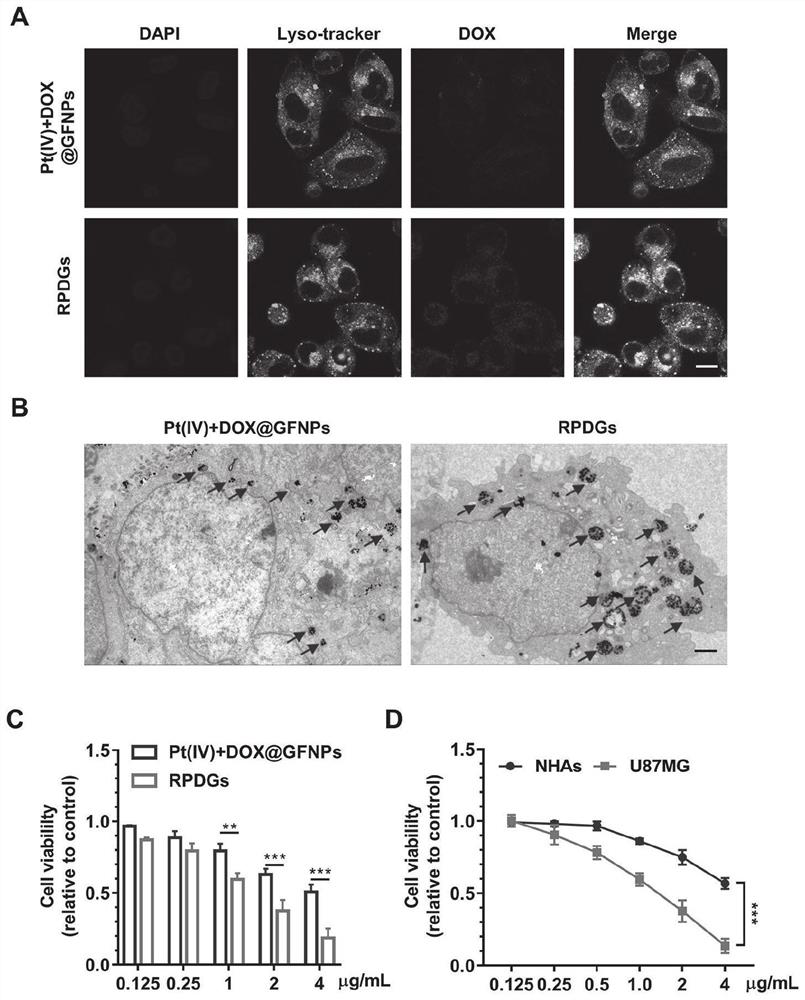
[0083] 2. Preparation of GA / Fe nanoparticles: 23mg FeCl 2 -4H 2 O and 80mg PVP were added to 4m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com