Benzimidazole compounds and their applications
A technology of benzimidazoles and compounds, applied in the field of biopharmaceuticals, can solve problems such as high toxicity and side effects, high treatment costs, and poor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound 1
[0034]
[0035] N, the preparation of 4-dimethyl-2-nitroaniline (A)
[0036] Add 4-methyl-2-nitroaniline (3g, 0.02mol), NaH (60%, 1.2g, 0.03mol), THF (50ml) in the 100ml round bottom flask, after stirring for 0.5h, add MeI (4.26g , 0.03mol), react overnight. Post-reaction treatment: After cooling the reaction system, add saturated brine and ethyl acetate, separate the layers, and wash the organic layer once with saturated brine. Purification by dry concentration and column chromatography (developing solvent: PE:EA=8:1) gave N,4-dimethyl-2-nitroaniline (A) with a yield of 91%.
[0037] N1, the preparation of 4-dimethyl-o-phenylenediamine (B)
[0038] Add N,4-dimethyl-2-nitroaniline (A) (1 g, 6 mmol), Pd / C (0.1 g), MeOH (30 ml) into a 100 ml round-bottomed flask and react overnight with hydrogen. Post-reaction treatment: Pd / C was removed by filtration, and the filtrate was dried and concentrated to obtain Compound B with a yield of 95%. ...
Embodiment 2
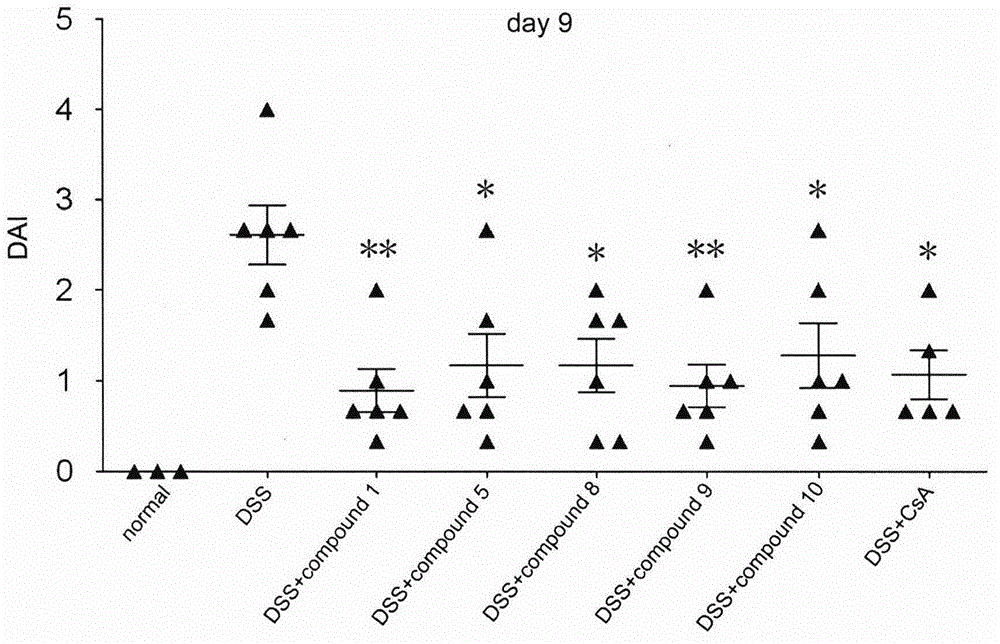
[0142] 1. Pharmacological tests of benzimidazole compounds
[0143] 1) Proliferation test of mouse T cells activated by anti-CD3 / anti-CD28
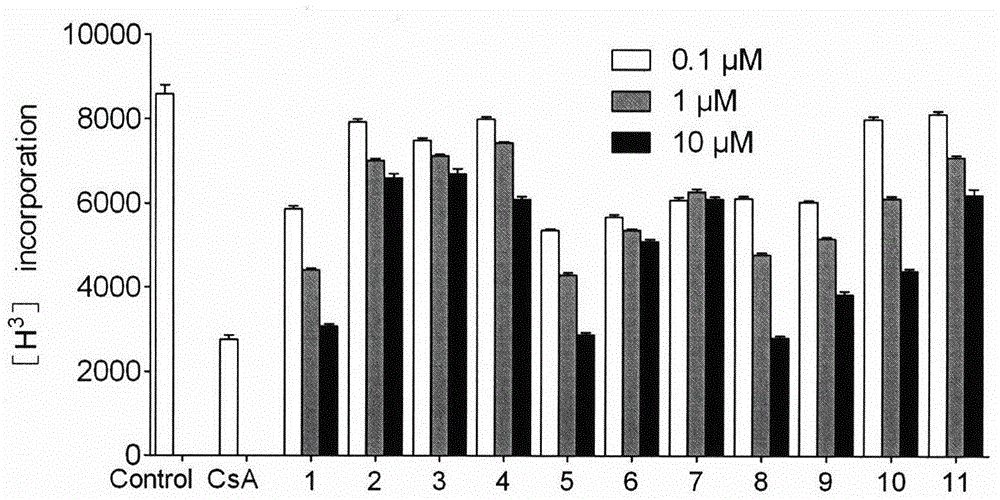
[0144] Four lymph nodes in the groin and armpit of the mice were taken to prepare a single cell suspension. Make 3×10 with complete medium RPMI-1640 6 / ml of cell suspension, seeded in 96-well plate, 3×10 5 Cells per well were stimulated with a final concentration of 10 μg / ml anti-CD3 and 1 μg / ml anti-CD28 (purchased from BD PharMingen) for 72 hours, incubated with compounds, and the proliferation of lymphocytes was detected by isotope tritium labeling.
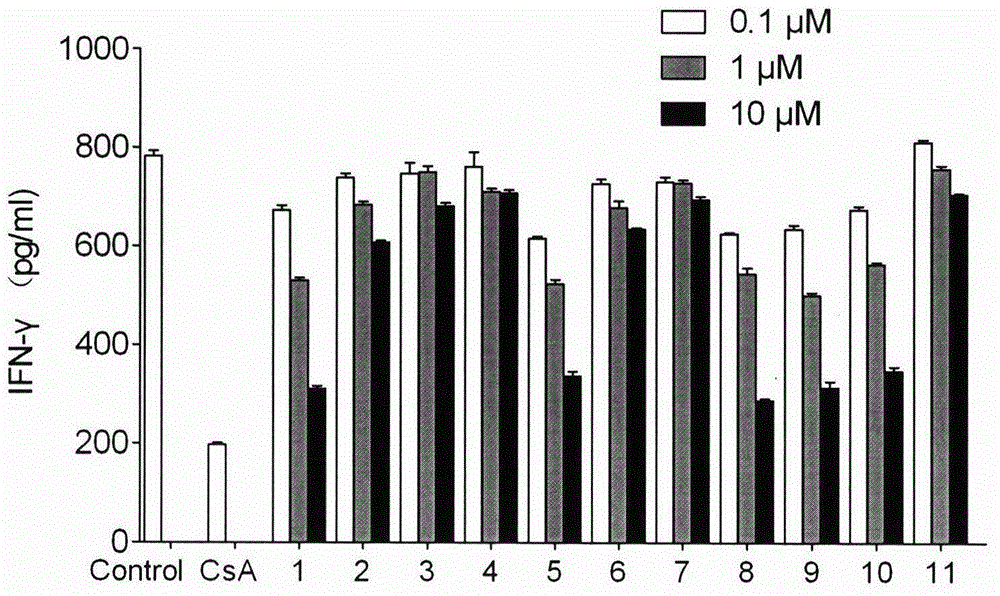
[0145] 2) Anti-CD3 / anti-CD28 activated mouse T cells secreted cytokine IFN-γ test
[0146] Four lymph nodes in the groin and armpit of the mice were taken to prepare a single cell suspension. Make 3×10 with complete medium RPMI-1640 6 / ml of cell suspension, seeded in 96-well plate, 3×10 5 Cells per well were stimulated with a final concentration of 10 μg / ml anti-CD3 and 1 μg / ml an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com