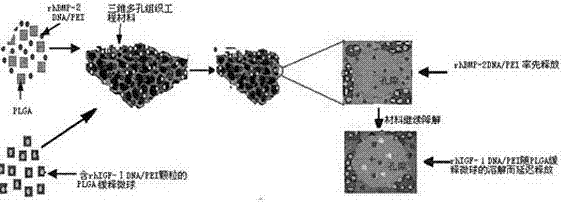
Preparation method of double-gene time sequence sustained-release tissue engineering scaffold material
A tissue engineering scaffold, dual-gene technology, applied in gene therapy, pharmaceutical formulations, genetic material management system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Through plasmid construction and amplification, 0.1g rhBMP-2 and 0.1g rhIGF-I are obtained.
[0032] Step 2. Preparation of rhBMP-2DNA / polyacetimide nanoparticles:
[0033] a. Mix 0.1grhBMP-2DNA and 30g polyethylene oxide uniformly to obtain a drug mixture,
[0034] b. Dissolve the drug mixture in 50mL N,N-dimethylformamide to obtain a drug oil solution;
[0035] c. Add the drug oil solution to 90g of a polyacetimide ethanol solution with a mass concentration of 60% and a weight average molecular weight of 1000 and continue to stir for 30 minutes;
[0036] d. Add 40g sucrose and lyophilize for later use.
[0037] Step 3. Preparation of rhIGF-Ⅰ DNA / polyacetimide nanoparticles;
[0038] a. Mix 0.1g rhIGF-I and 30g erythritol uniformly to obtain a drug mixture,
[0039] b. Dissolve the drug mixture in 50 mL of tetrahydrofuran to obtain a drug oil solution;
[0040] c. Add the drug oil solution to 90 g of a polyacetimide ethanol solution with a mass concentration of 60% and a wei...
Embodiment 2
[0049] Step 1: Through plasmid construction and amplification, 0.1g rhBMP-2 and 0.1g rhIGF-I are obtained.
[0050] Step 2. Preparation of rhBMP-2DNA / polyacetimide nanoparticles;
[0051] a. Mix 0.1grhBMP-2DNA and 30g polypyrrolidone uniformly to obtain a drug mixture,
[0052] b. Dissolve the drug mixture in 50 mL of thionyl chloride to obtain a drug oil solution;
[0053] c. Add the drug oil solution to 90 g of a polyacetimide solution with a mass concentration of 60% and a weight average molecular weight of 3500 and continue to stir for 40 minutes;
[0054] d. Add 40g of sucrose and freeze-dry it for later use;
[0055] Step 3. Preparation of rhIGF-Ⅰ DNA / polyacetimide nanoparticles;
[0056] a. Mix 0.1g rhIGF-I and 30g pharmaceutical excipient polyethylene oxide uniformly to obtain a drug mixture,
[0057] b. Dissolve the drug mixture in 50 mL of organic solvent ethyl acetate to obtain a drug oil solution;
[0058] c. Add the drug oil solution to 90g of a polyacetimide solution with a ma...
Embodiment 3
[0067] Step 1: Through plasmid construction and amplification, 0.1g rhBMP-2 and 0.1g rhIGF-I are obtained.
[0068] Step 2. Preparation of rhBMP-2DNA / polyacetimide nanoparticles;
[0069] a. Mix 0.1grhBMP-2DNA and 30g erythritol as a pharmaceutical excipient to obtain a drug mixture.
[0070] b. Dissolve the drug mixture in 50 mL of organic solvent thionyl chloride to obtain a drug oil solution;
[0071] c. Add the drug oil solution to 90 g of a polyacetimide solution with a mass concentration of 60% and a weight average molecular weight of 9000 and continue to stir for 50 minutes;
[0072] d. Add 40g of sucrose and freeze-dry it for later use;
[0073] Step 3. Preparation of rhIGF-Ⅰ DNA / polyacetimide nanoparticles;
[0074] a. Mix 0.1g rhIGF-I and 30g polyethylene oxide uniformly to obtain a drug mixture,
[0075] b. Dissolve the drug mixture in 50mL N,N-dimethylformamide to obtain a drug oil solution;
[0076] c. Add the drug oil solution to 90 g of a polyacetimide solution with a mass co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com