Porous tissue scaffoldings for the repair or regeneration of tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
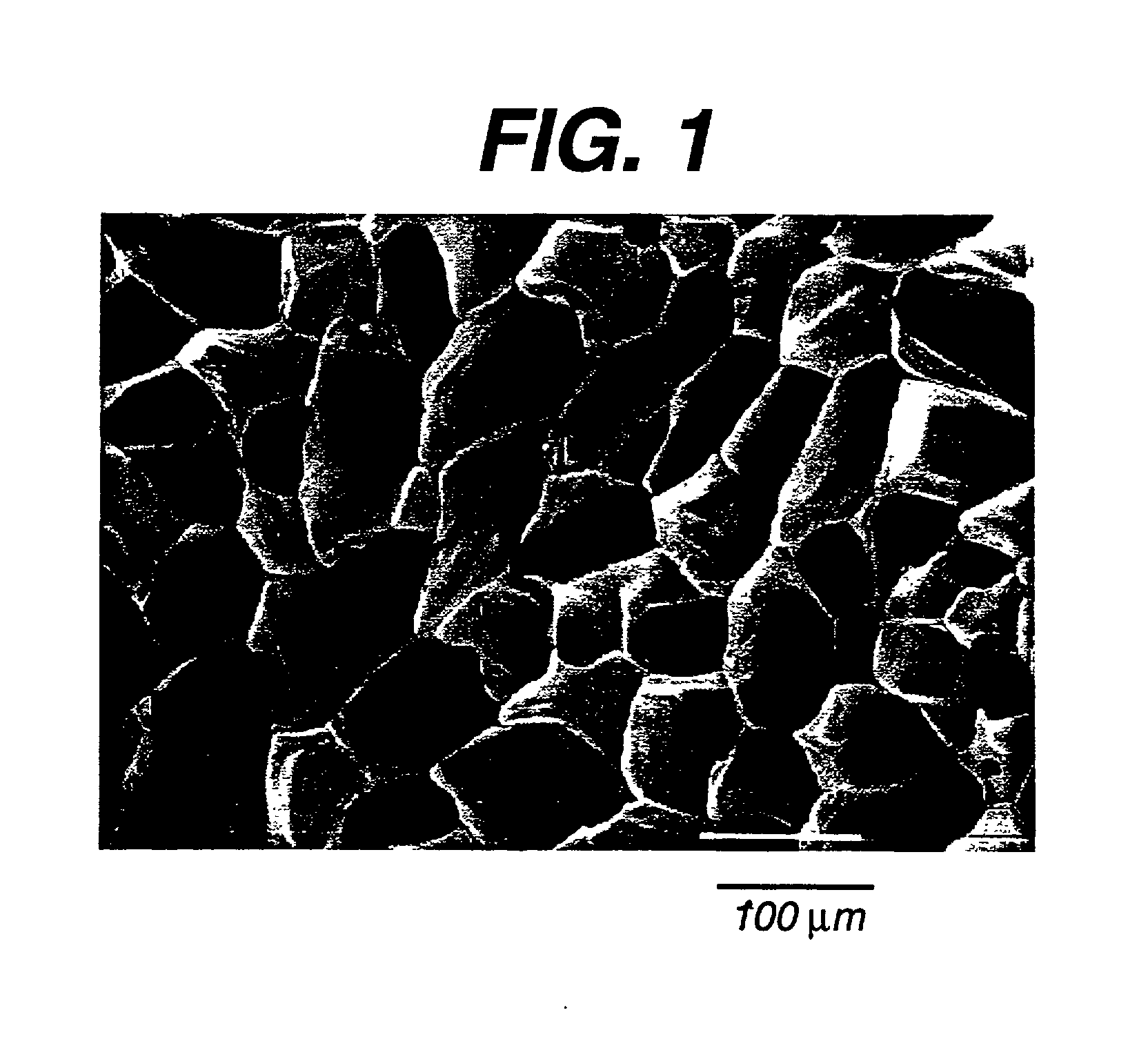
[0098] Preparation of a Foam With Random Microstructure (No Preferred Architecture)
[0099] Step A. Preparing 5% wt. / wt. Homogeneous Solution of 35 / 65 PCL / PGA in 1,4-Dioxane
[0100] A 5% wt. / wt. polymer solution is prepared by dissolving 1 part of 35 / 65 PCL / PGA with 19 parts of the solvent 1,4-dioxane. The 35 / 65 PCL / PGA copolymer was made substantially as described in Example 8. The solution is prepared in a flask with a magnetic stir bar. For the copolymer to dissolve completely, it is recommended that the mixture is gently heated to 60.+-.5.degree. C. and continuously stirred for a minimum of 4 hours but not exceeding 8 hours. A clear homogeneous solution is then obtained by filtering the solution through an extra coarse porosity filter (Pyrex brand extraction thimble with fritted disc) using dry nitrogen to help in the filtration of this viscous solution.
[0101] Step B. Lyophilization
[0102] A laboratory scale lyophilizer--Freezemobile 6 of VIRTIS was used in this experiment. The freez...
example 2
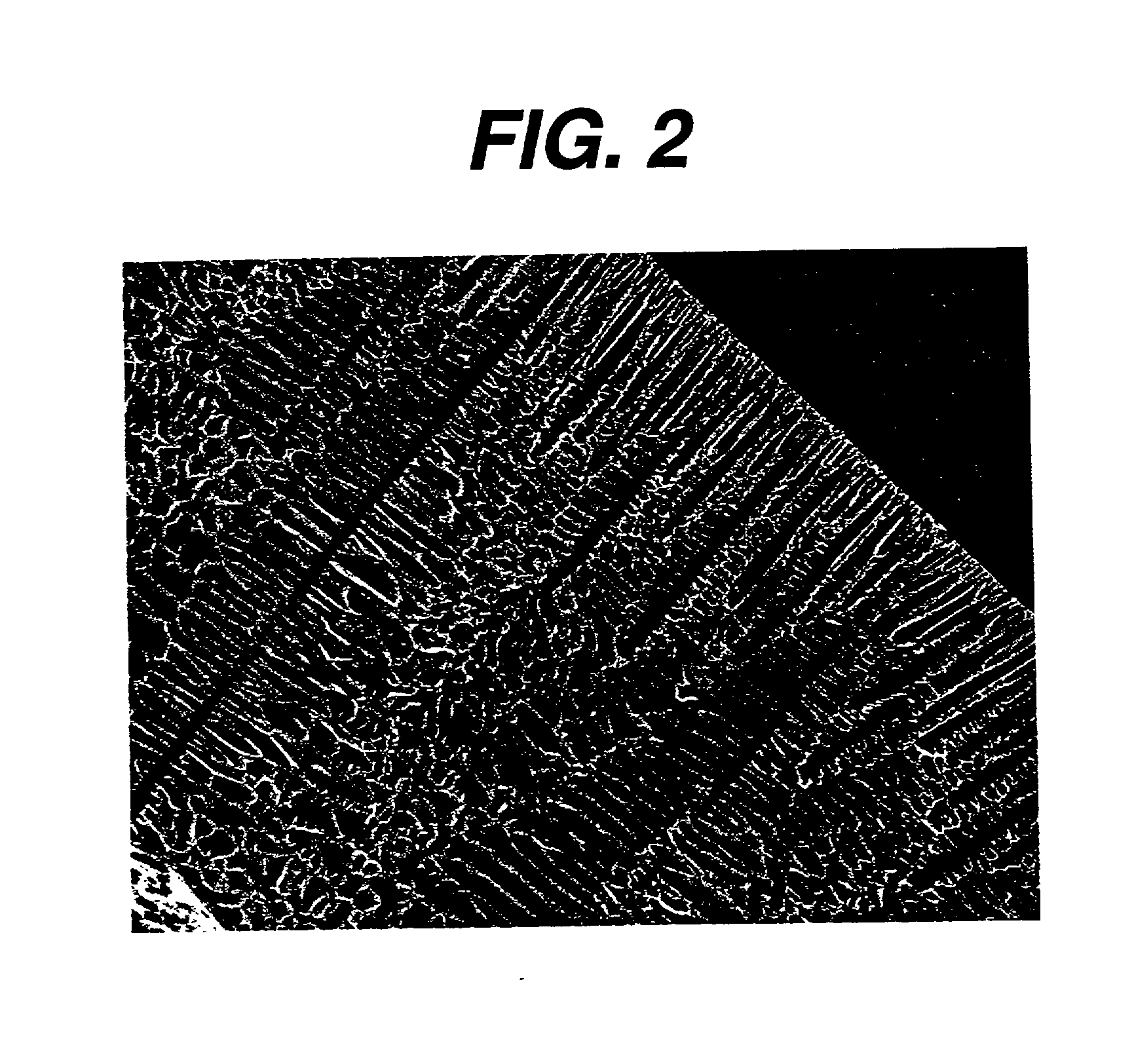
[0109] Preparation of a Foam With Vertical Channels
[0110] This example describes the making of a 35 / 65 PCL / PGA foam with vertical channels that would provide pathways for nutrient transport and guided tissue regeneration.
[0111] We used a FTS Dura Dry Freeze dryer with computer control and data monitoring system to make this foam. First step in the preparation of this foam was to generate a homogeneous solution. A 10% wt. / wt. homogeneous solution of 35 / 65 PCL / PGA was made in a manner similar to that described in Example 1, Step A. The polymer solution was carefully filled into a dish just before the actual start of the cycle. The dish weighed 620 grams, was optical glass 5.5 mm thick, and cylindrical with a 21 cm outer diameter and a 19.5 cm inner diameter. The lip height of the dish was 2.5 cm. Next the following steps are followed in sequence to make a 2 mm thick foam with the desired architecture:
[0112] (i). The solution filled dish was placed on the freeze dryer shelf that was pr...
example 3
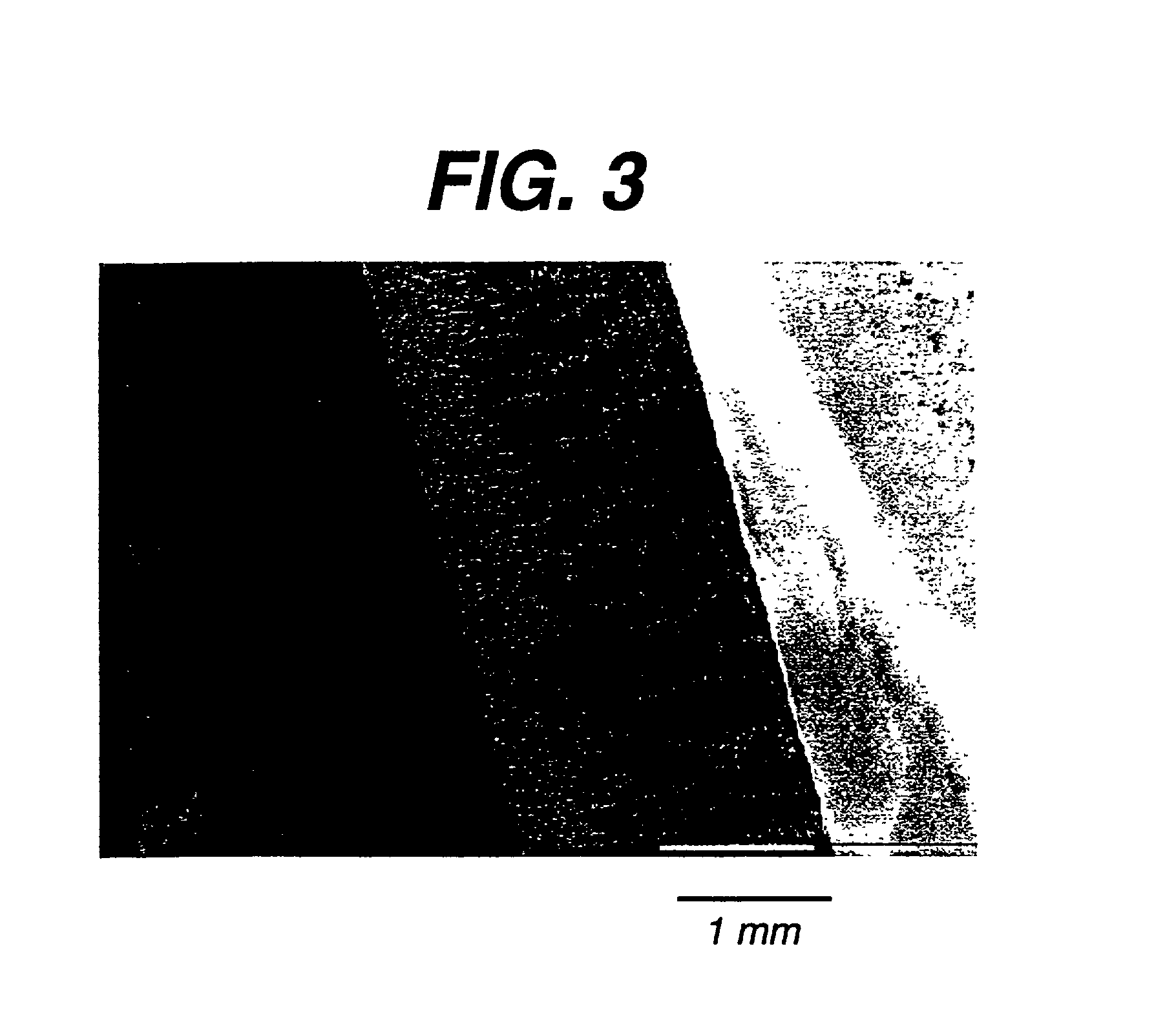
[0117] Architecturally Gradient Foam
[0118] This example describes the making of a foam that has a gradient in foam morphology as shown in FIG. 3 using a 10% solution of 35 / 65 .epsilon.-caprolactone-co-glycolide. The method used to make such a foam is similar to the description given in Example 2 with one difference. In step (ii) of the lyophilization process the time for which the solution is kept at the freezing step is 30 minutes.
[0119] FIG. 3 is a scanning electron micrograph of a cross section of this foam. Note the variation in the pore size and pore shape through the thickness of the foam.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com