Use of iso-glycyrrhizic acid and salt thereof in treating allergic rhinitis
A technology for allergic rhinitis and isoglycyrrhizic acid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
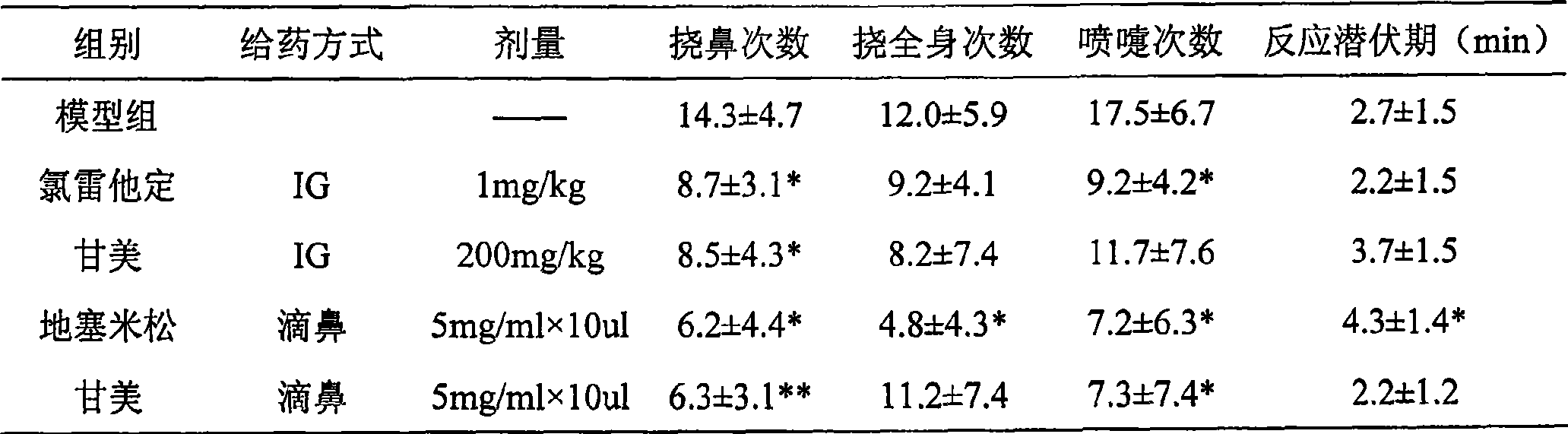
[0017] 1. Experimental materials:
[0018] 1.1 Animals:
[0019] Wistar rats, male, 200-300 g. Provided by the Experimental Animal Center of the Academy of Military Medical Sciences of the Chinese People's Liberation Army, SCXK-(Army) 2002-001.
[0020] 1.2 Reagents:
[0021] ①. Calf serum albumin: provided by Nanjing Shengxing Company.
[0022] ② Complete Freund's adjuvant: provided by sigma company.
[0023] ③. Magnesium isoglycyrrhizinate (lummy): Provided by CTTQ company, and the required concentration was prepared with NS.
[0024] ④. Loratadine syrup: Provided by Schering-Plough, Belgium, 6ANNA56001, 200610, 1 mg / ml, prepared with NS at a suitable concentration for intragastric administration, with a dose of 1 mg / kg.
[0025] ⑤. Dexamethasone Sodium Phosphate Injection: Guizhou Huasheng Pharmaceutical Co., Ltd., 050103, prepared to a concentration of 5 mg / ml, and the intranasal administration dose of 10 μl / animal.
[0026] 1.3 Antigen suspension preparation:
[00...
Embodiment 2
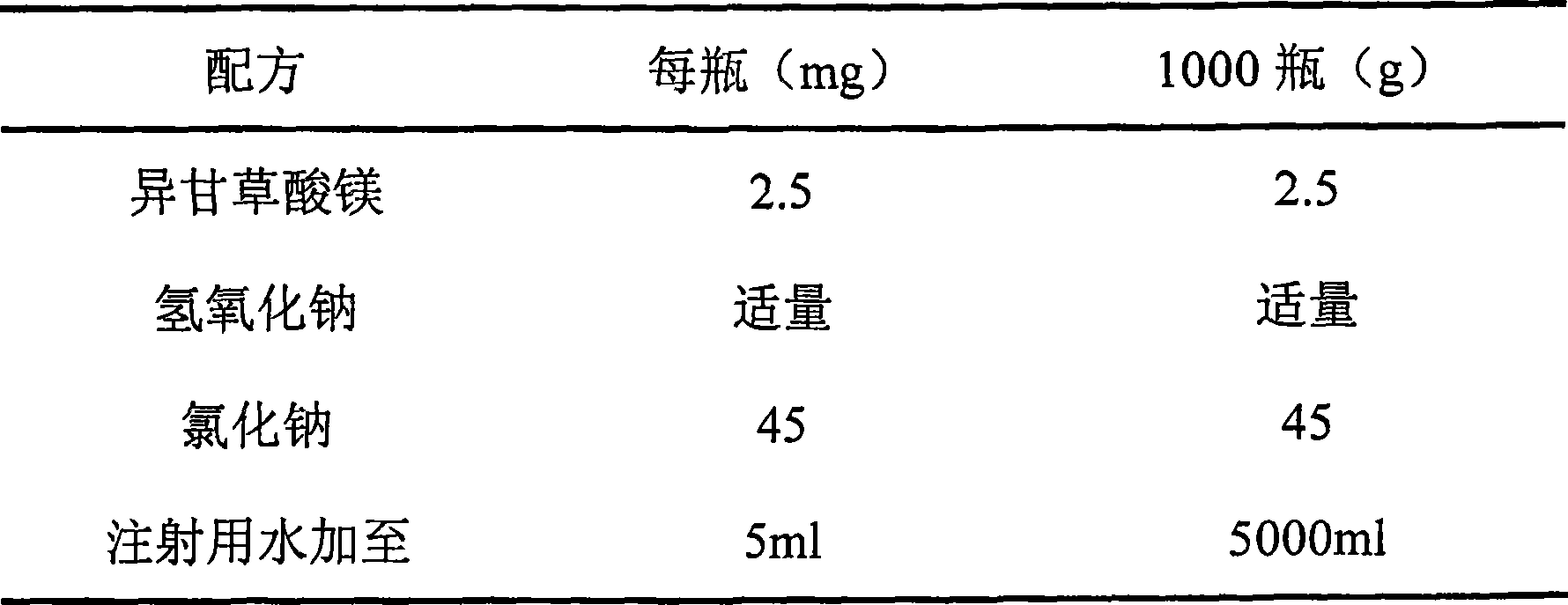
[0040] Example 2 Magnesium Isoglycyrrhizinate Nasal Drops
[0041] prescription:
[0042]
[0043] Preparation:
[0044] 1) Solution preparation
[0045] Add magnesium isoglycyrrhizinate and sodium chloride into 4000ml of water for injection, stir to form a uniform suspension, adjust the pH value to 6.0 with NaOH, stir continuously to dissolve the main ingredient, add water for injection to a sufficient amount, and pass through a 0.22 μm filter membrane to obtain intermediate.
[0046] 2) Packing
[0047] After passing the inspection, the intermediates are aseptically divided into bottles according to the content of the intermediates and sealed with thermoplastic caps.
Embodiment 3
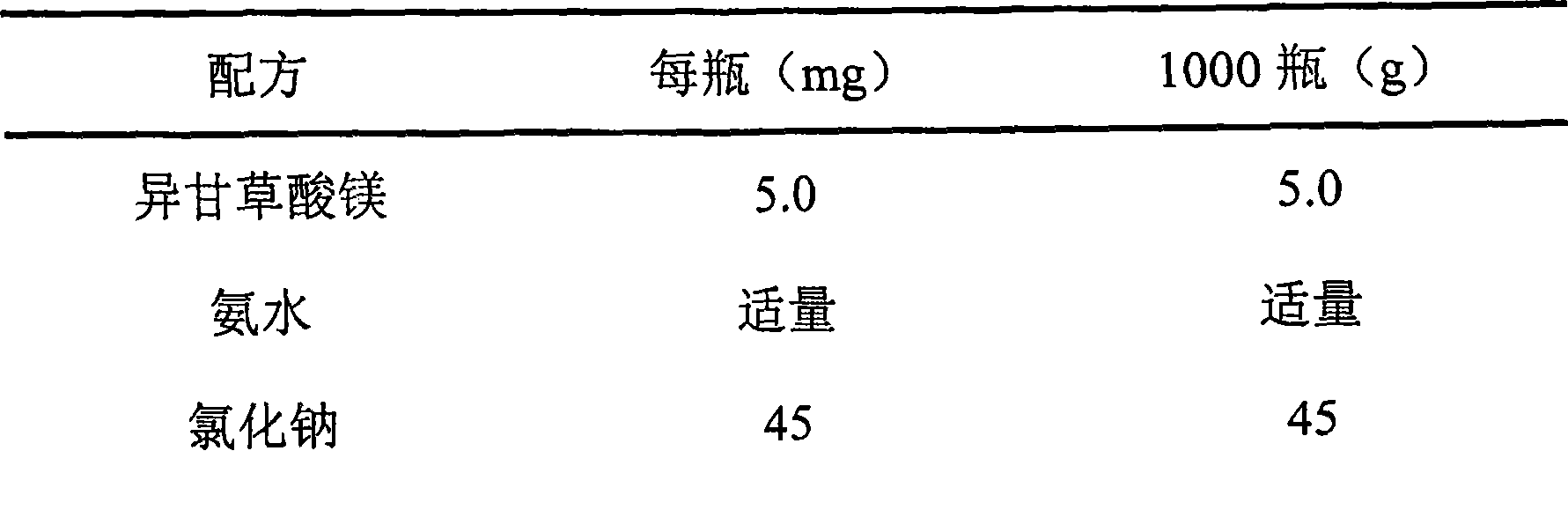
[0048] Example 3 Magnesium Isoglycyrrhizinate Nasal Drops
[0049] prescription:
[0050]
[0051]
[0052] Preparation:
[0053] 1) Solution preparation
[0054] Add magnesium isoglycyrrhizinate and sodium chloride into 4000ml of water for injection, stir to form a uniform suspension, adjust the pH value to 7.0 with ammonia water, stir continuously to dissolve the main ingredient, add water for injection to a sufficient amount, and pass through a 0.22 μm filter membrane to obtain intermediate.
[0055] 2) Packing
[0056] After passing the inspection, the intermediates are aseptically divided into bottles according to the content of the intermediates and sealed with thermoplastic caps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com