Composition and pharmaceutical dosage form for colonic drug delivery using polysaccharides
A composition and drug technology, applied in the directions of drug combination, sugar-coated pills, drug delivery, etc., can solve the problems of colon enzymes cannot easily reach polysaccharides, cannot display colon-specific drug delivery, slow drug release, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0055] EXAMPLES Example 1: Preparation of a film made from the composition
[0056] Pectin and guar gum (4:1 w / w) were mixed and dissolved in distilled water to a final concentration of 2% (w / v). Dilute aliquots with Na 2 CO 3 The pH of the mixture was adjusted to 4, 5, 6, 7, 8, and 10. Each was cast on a polytetrafluoroethylene plate to form a film with a thickness of 150 µm.
[0057] Some other samples were also prepared in which locust bean gum was substituted for guar gum and the pH of the samples was adjusted to 4 and 8. The film is cast and dried. The thickness of these dried films was 150 μm.
[0058] Some other samples were also prepared in which pectin was dissolved in distilled water with Na 2 CO 3 Adjust pH to 4 and 8. The samples were cast and dried. The thickness of these dried films was also 150 μm.
[0059] Finally, guar gum and locust bean gum were used at pH 7 and 14 to prepare films as described above. The thickness of these dried films was also 15...
Embodiment 2
[0063] Table 2
[0064] The results show that the prepared membranes prepared at pH around 7 or above have better tonic properties compared to the prepared membranes prepared at pH below 7. Embodiment 3: with this composition tablet coating
Embodiment 3
[0064] The results show that the prepared membranes prepared at pH around 7 or above have better tonic properties compared to the prepared membranes prepared at pH below 7. Embodiment 3: with this composition tablet coating
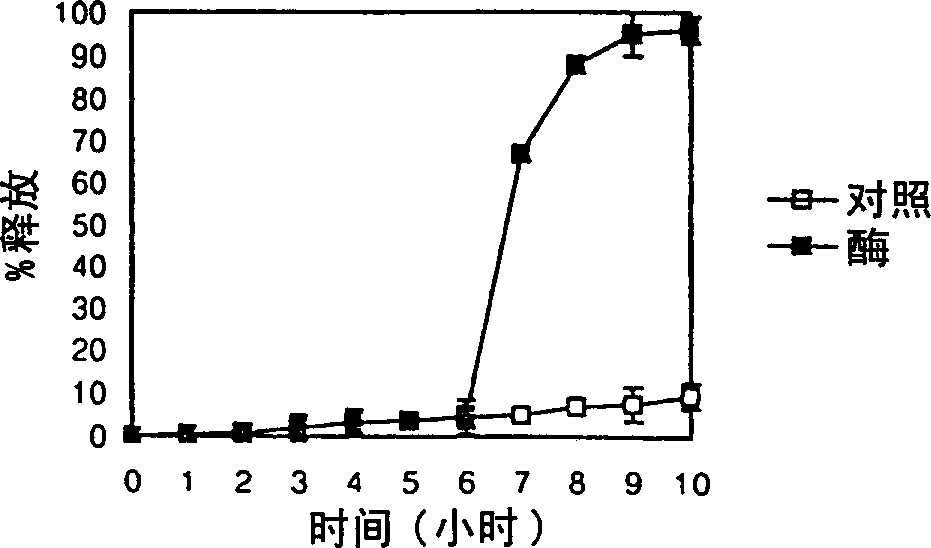
[0065] Tablets containing 100 mg of ibuprofen as a model drug were coated with the composition prepared according to the method of Example 1. The weight ratio of pectin to guar gum was 4:1, and the pH was adjusted to 8. Coat with Hi-Coater (HCT-MINI, Freund Ind., Japan), coating amount is 8mg / cm 2 ,15mg / cm 2 ,26mg / cm 2 , and 35mg / cm 2 .
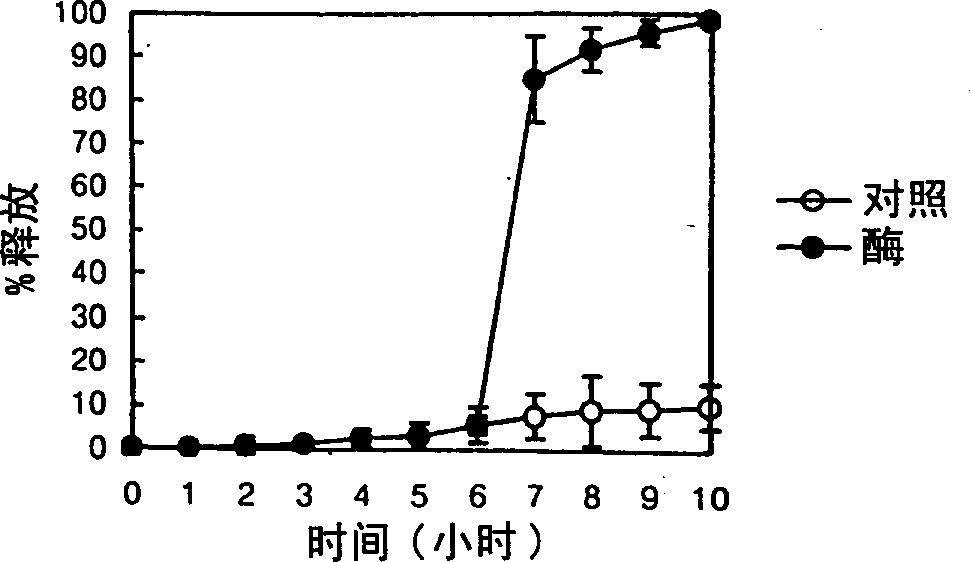
[0066] Another tablet containing 100 mg of ibuprofen as a model drug was coated with the composition prepared according to the method of Example 1, wherein locust bean gum was used instead of guar gum. The weight ratio of pectin to locust bean gum was 4:1, and the pH was adjusted to 8. Coat with Hi-Coater (HCT-MINI, Freund Ind., Japan), coating amount is 15mg / cm 2 .
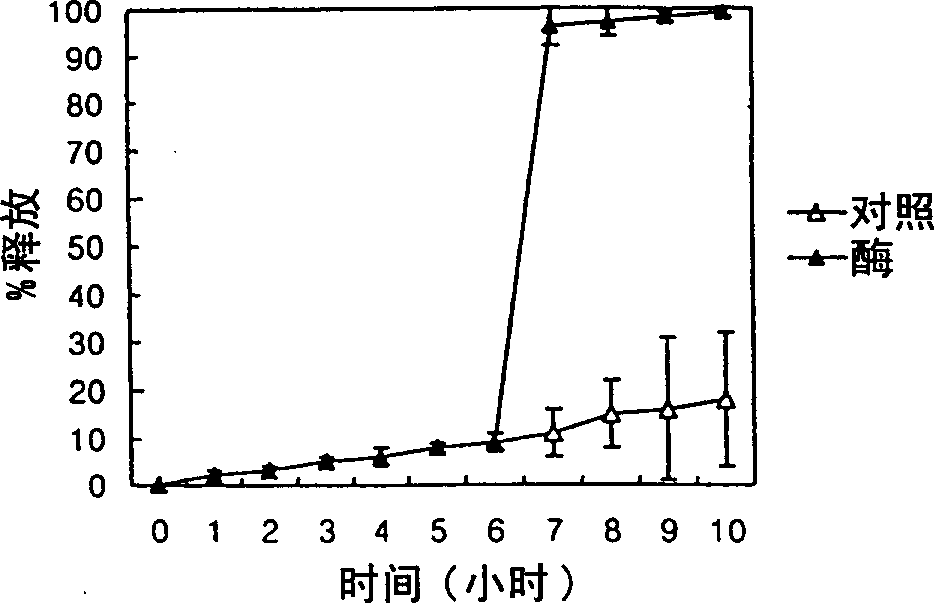
[0067] Control preparations containing 100 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com