Novel application of eye medicine wrapped with erythrocyte membrane
A technology of red blood cell membranes and uses, which is applied in the field of preparation of ophthalmic preparations, can solve the problems of inconvenient use, etc., and achieve the effects of prolonging residence time, reducing systemic side effects, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Latanoprost-PCL Nanoparticles Wrapped in Erythrocyte Membrane in Example 1
[0031] Preparation of Latanoprost-Polycaprolactone (PCL) Nanoparticles by Emulsification
[0032] The specific method is: 440ug of latanoprost is dissolved in chloroform containing 4 mg of PCL ethanol solution. The mixture was sonicated and emulsified in 2 mL of water containing 0.25% (wt / wt) polyvinyl alcohol (PVA). After evaporating overnight in an organic solvent, the residue was washed in an Amicon 100k MWCO filter to remove PVA to obtain PCL nanoparticles. The size and dispersibility of the PCL particles were detected by a dynamic light scattering instrument.
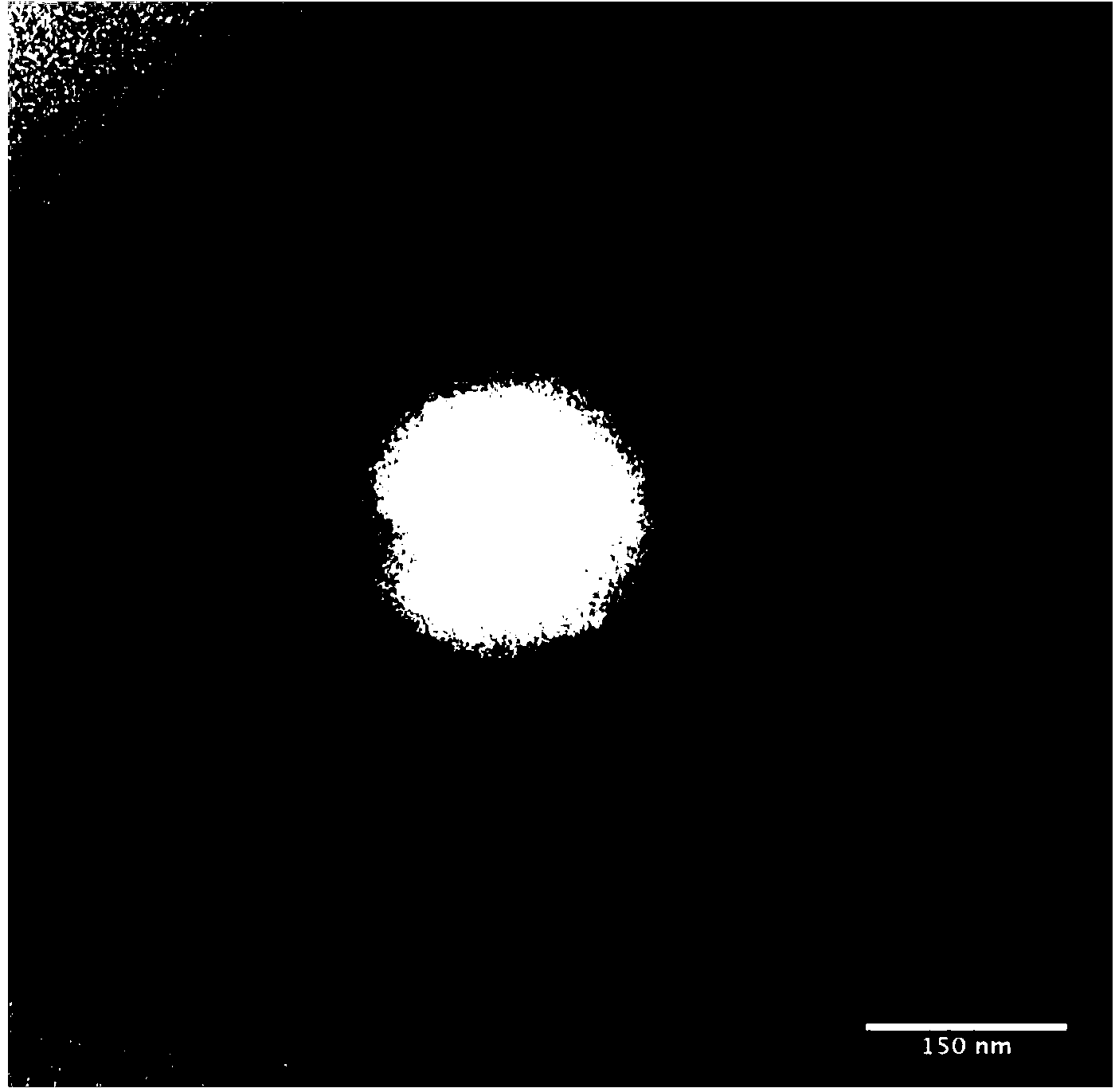
[0033] Mix the red blood cell membrane with the above-mentioned prepared nanoparticles, and then extrude the mixture through a 400-nanometer porous membrane, and the resulting particles are latanoprost-PCL nanoparticles wrapped in red blood cell membranes, with an average diameter of 350 nanometers (See the transmission electron m...
Embodiment 2
[0035] Example 2 Latanoprost-PLGA nanoparticles wrapped in erythrocyte membrane
[0036] Preparation of Latanoprost-Polycaprolactone (PLGA) Nanoparticles by Emulsification
[0037] The specific method is:
[0038] First, the PLGA polymer was dissolved in acetone at a concentration of 1 mg / ml, and latanoprost was dissolved in the PLGA solution at 1 mM, and then 1 ml of the solution was added dropwise to 3 ml of water, and the resulting mixture was exposed to air and stirred for 2 hours After centrifugation or filtration, latanoprost PLGA nanoparticles can be obtained.
[0039] The erythrocyte membrane is mixed with the above-prepared nanoparticles, and then the mixture is extruded through a 400-nanometer porous membrane, and the resulting particles are latanoprost-PLGA nanoparticles wrapped in the erythrocyte membrane.
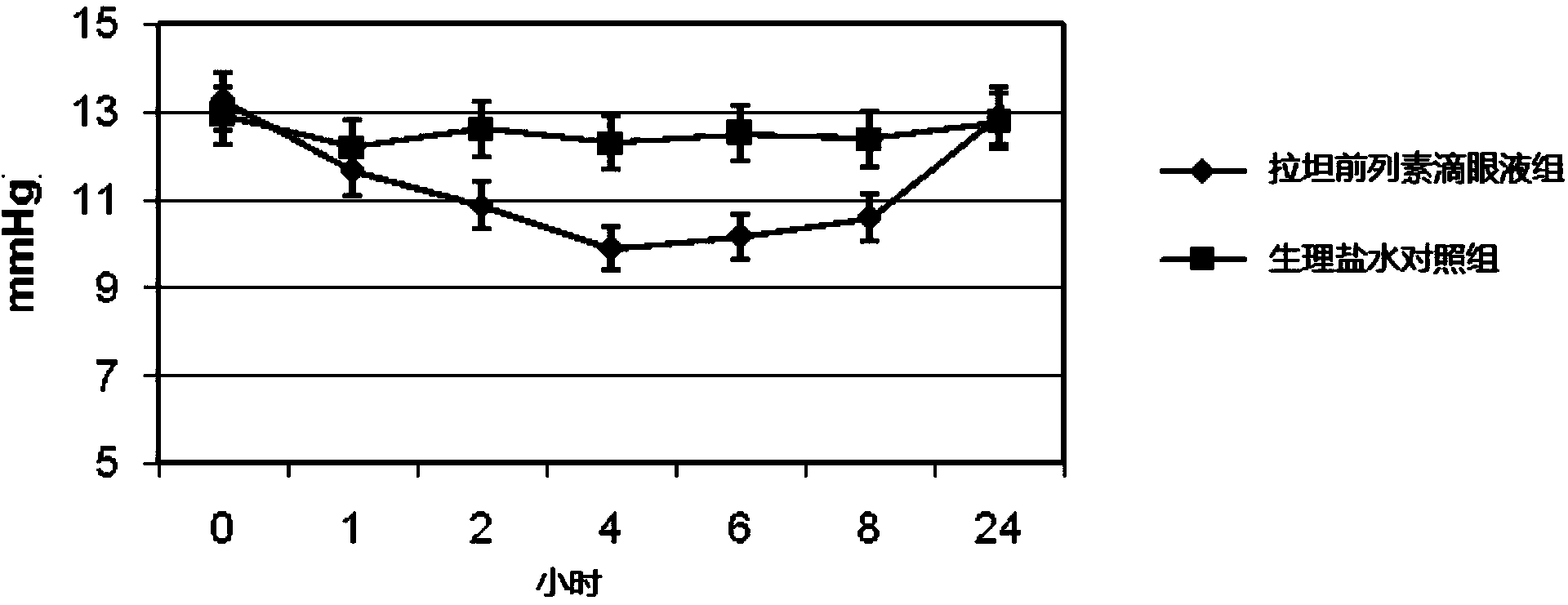
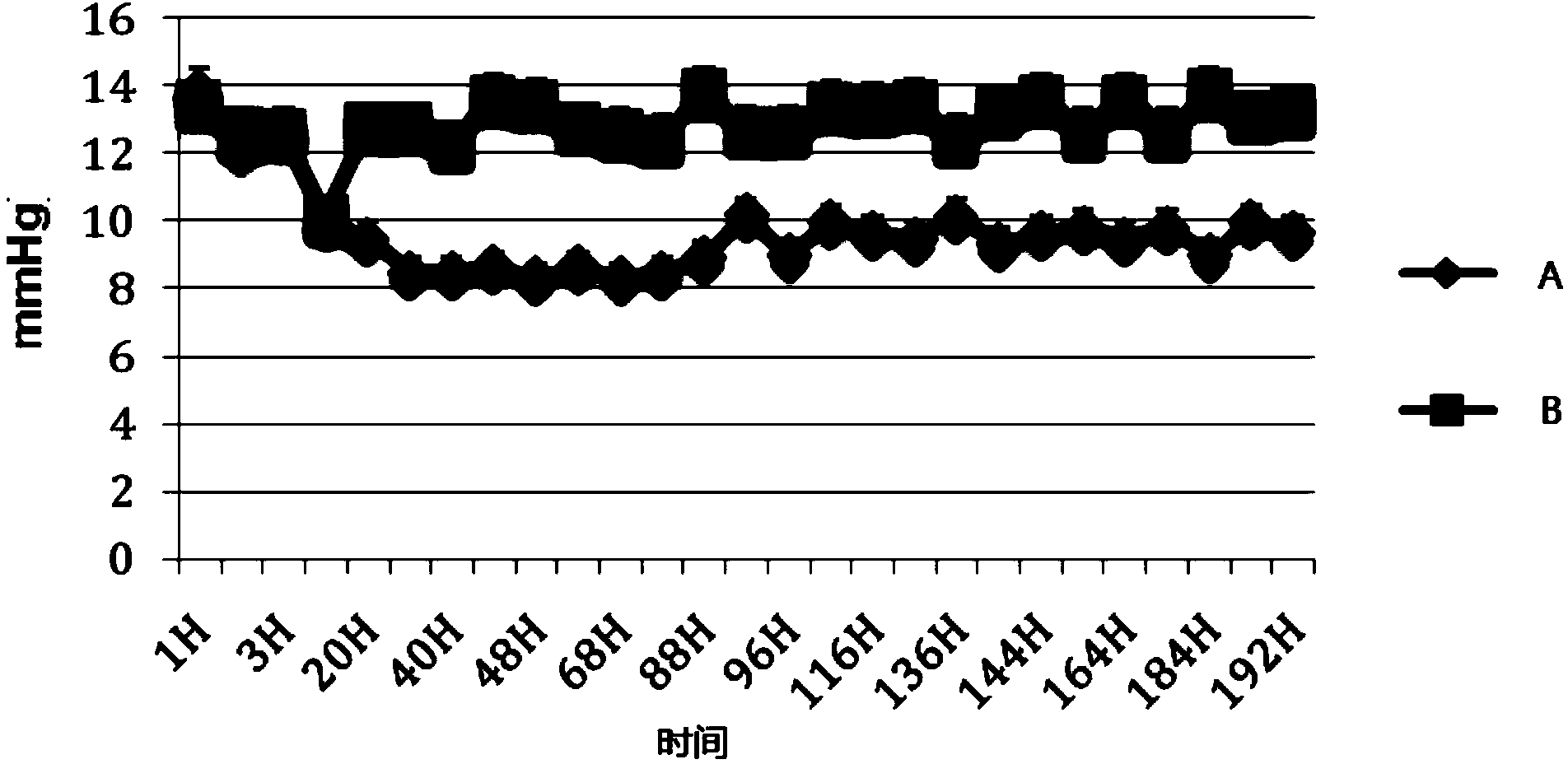
[0040] The beneficial effects of the present invention will be specifically described below through test examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com