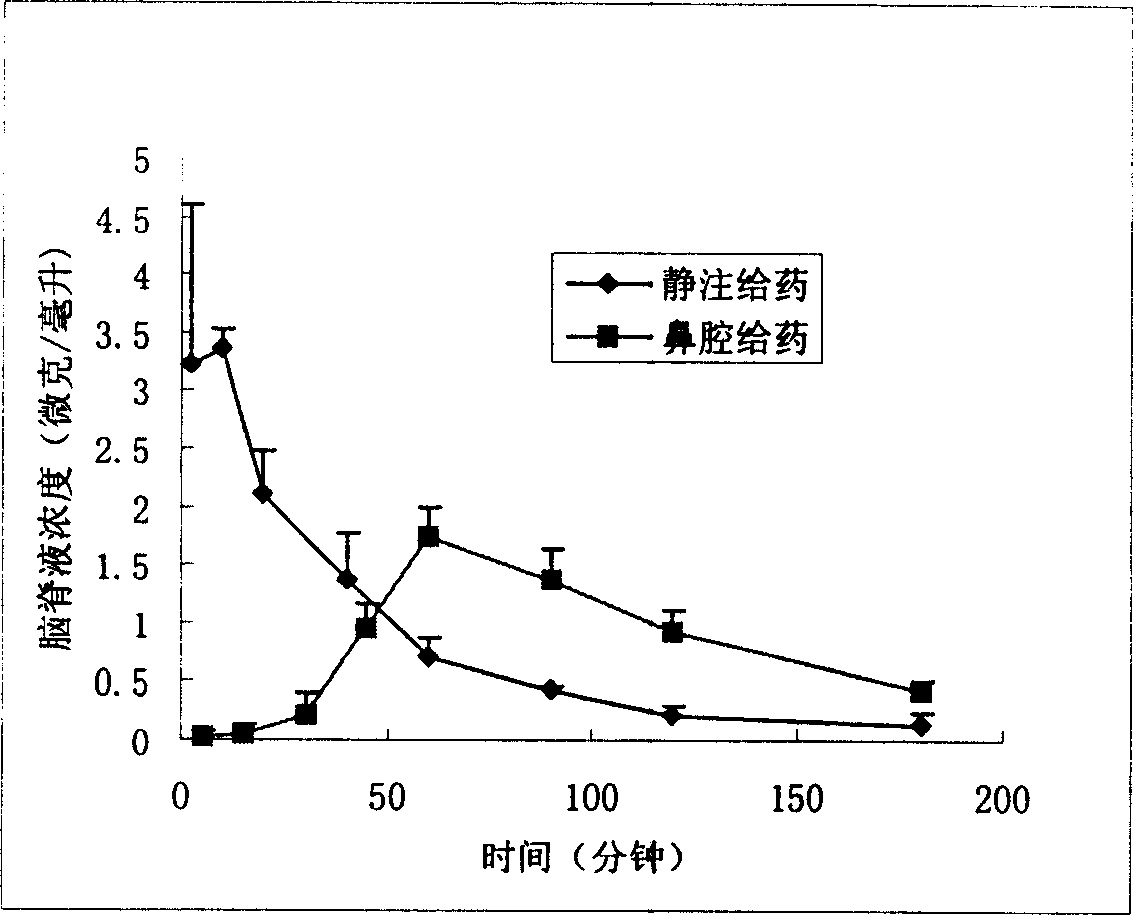
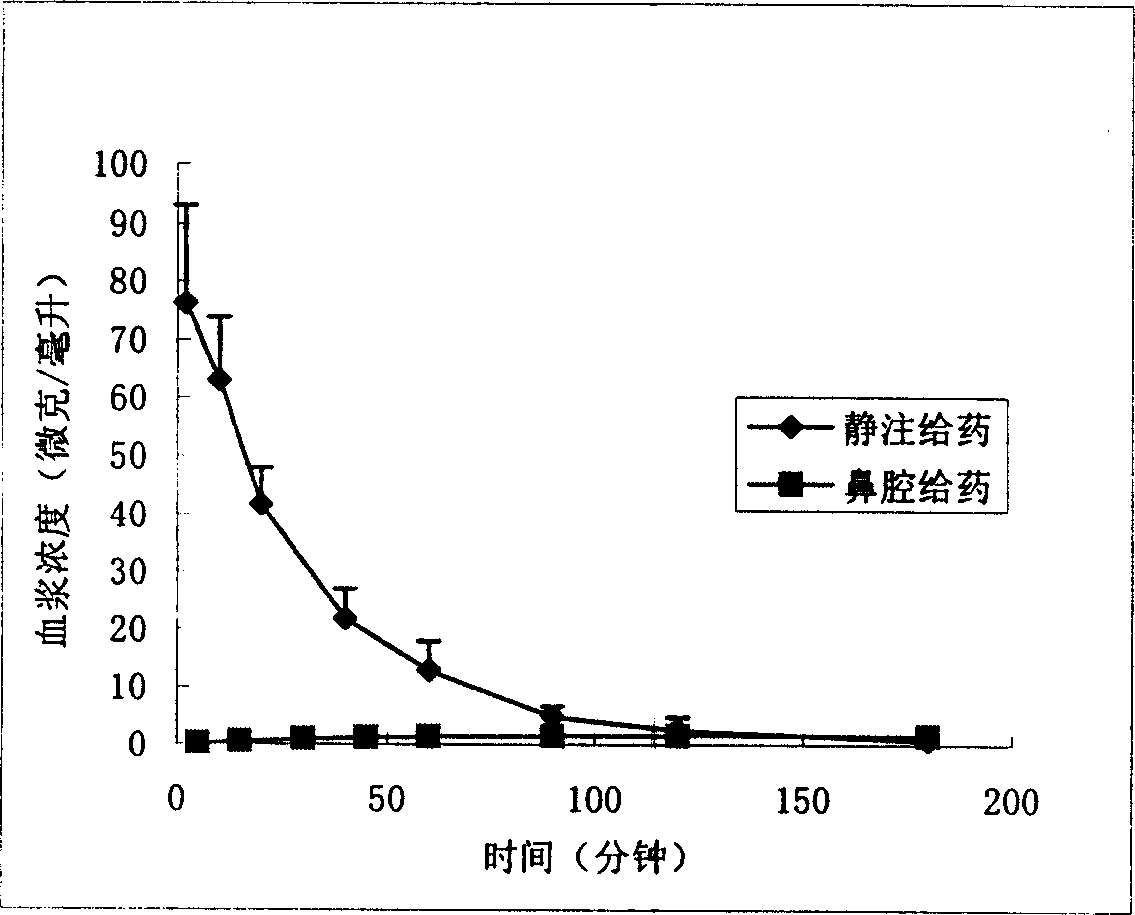
Nose cavity administering formulation of gastrodine
A nasal administration preparation and a technique for intranasal administration, which are applied in the field of gastrodin nasal administration preparations and can solve the problems of increasing patient pain and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Gastrodin 30g
[0026] borneol 0.3g
[0027] Hydroxypropyl Beta Cyclodextrin 6g
[0028] Distilled water up to 100ml
[0029] Preparation method: Put borneol and β-cyclodextrin in water, dissolve them with ultrasonic waves, and then dissolve gastrodin in the above solution to obtain the product.
[0030] Rats were anesthetized, tracheal intubation, esophageal ligation, put on a stereotaxic instrument, maintained body temperature at 37°C with a heating pad, trimmed and disinfected the head and neck, made a longitudinal incision along the longitudinal axis with a scalpel, and bluntly separated the neck with scissors Dorsal muscles, exposing the foramen magnum. According to the weight of the rat, insert the sampling needle from the foramen magnum into the cerebellar cisterna magna to collect the cerebrospinal fluid. After the cerebrospinal fluid can flow out due to the difference in internal and external pressure, fix the sampling needle with glue, turn the rat over, an...
Embodiment 2
[0036] Gastrodin 9g
[0037] Polysaccharide gellan gum 0.03g
[0038] Ethylparaben 0.02g
[0039] Distilled water up to 30ml
[0040] Preparation method: Swell gellan gum in distilled water, then add gastrodin, mix well, put it into a nasal sprayer, spray 70 μl each time, and spray once in each of the left and right nostrils.
Embodiment 3
[0042] Gastrodin 15g
[0043] Glyceryl monostearate 7g
[0044] Stearic acid 10g
[0045] White Vaseline 6g
[0046] Liquid paraffin 40ml
[0047] ice flakes 2g
[0048] Sodium Lauryl Sulfate 1g
[0049] Ethylparaben 0.1g
[0050] Distilled water 30ml
[0051] Preparation method: Take glyceryl stearate, stearic acid, white vaseline and liquid paraffin and heat and melt to form an oil phase. Add borneol to the oil phase to dissolve. In addition, distilled water was heated to 80°C, sodium lauryl sulfate and ethyl paraben were added to dissolve, and then gastrodin was added to dissolve into an aqueous phase. Then slowly pour the water phase into the oil phase and stir until it condenses.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com