Preparation method of lenvatinib mesylate crystal form X
A technology of lenvatinib and methanesulfonic acid, which is applied in the field of pharmaceutical crystal forms, can solve the problems of undisclosed, base toxic impurities and alcoholysis impurities exceeding the standard, undisclosed crystal form X, etc., to achieve improved yield, simple operation, The effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0042] Specific implementation method: screening test
[0043] In order to avoid the impact of alcoholysis impurities and mesylate impurities on product quality and to avoid the use of alcoholic solvents, the inventors of the present application carried out the solvent types and combinations used in step (1) in the process of obtaining the technical solution. A large number of screens were conducted, and data statistics were carried out on the crystal form of the final product and related substances. The results are shown in Table 1 below.
[0044] Table 1 Experimental results for the preparation of Form X in different solvent systems
[0045] solvent system crystal form alcoholysis impurities Mesylate impurity Methanol Form A 0.17% 97ppm ethanol Form A 0.15% 89ppm Isopropanol Form A 0.13% 91ppm acetone Form A 0 23ppm ethyl acetate Form A 0 21ppm Acetonitrile Form A 0 17ppm methanol and water Form...
Embodiment 1
[0048] Embodiment 1: Preparation of lenvatinib mesylate crystal form X
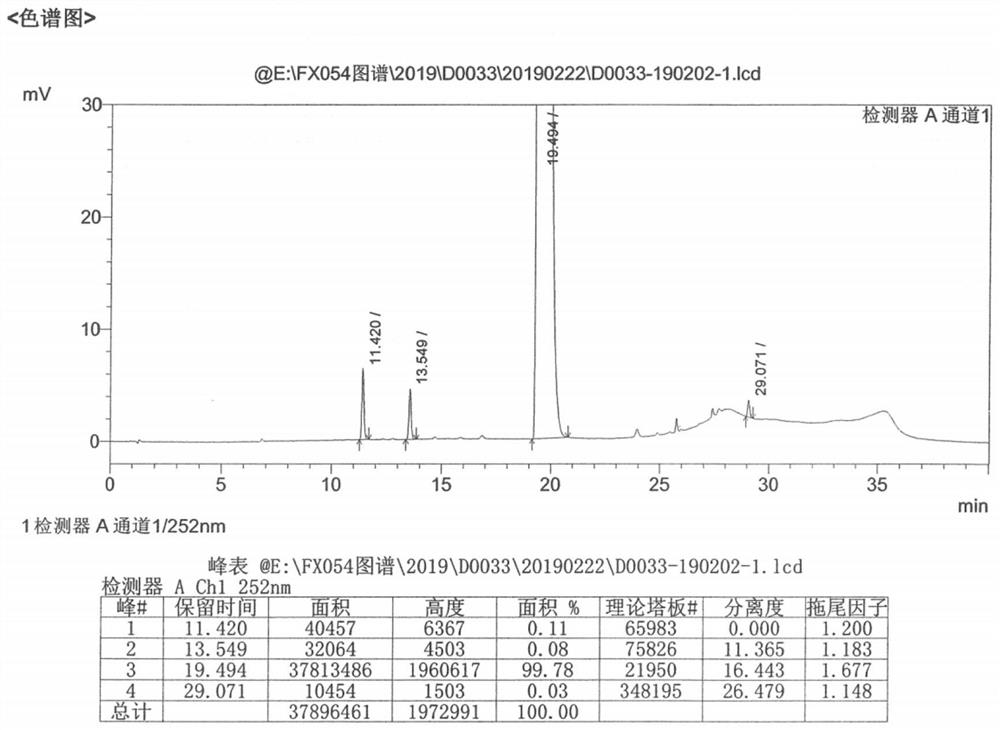
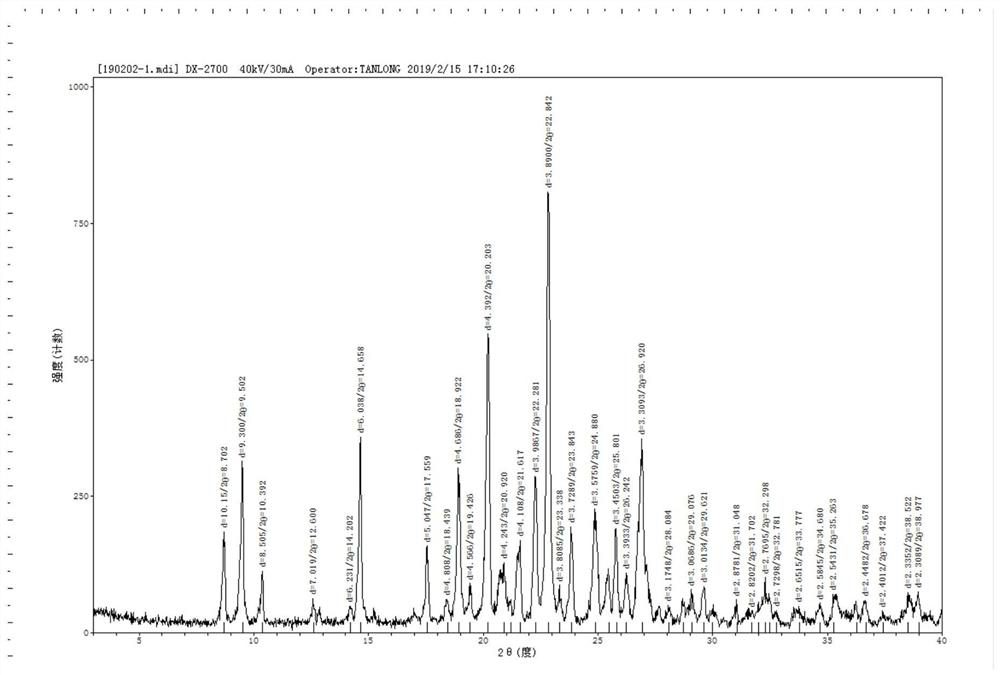
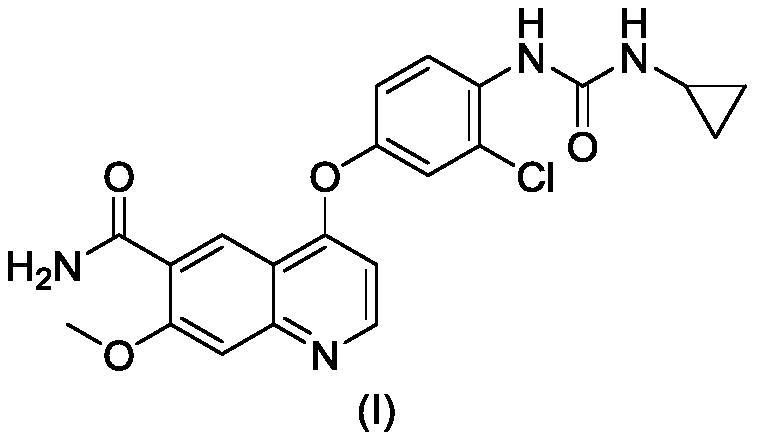
[0049] Disperse 10.0g of lenvatinib in 20ml of purified water and 30ml of acetonitrile, stir and beat at 30°C to form a suspension; then add the mixed solution of 2.7g of methanesulfonic acid, 10ml of acetic acid and 20ml of acetonitrile into the suspension; After the dropwise addition was complete and the suspension was dissolved, 3% seed crystal X was added, and then 20ml of acetonitrile was added dropwise to the suspension. After the dropwise addition, the mixture was stirred at 25°C for 12h, filtered and dried to obtain 8.93g of solid powder. Yield 89.3%. The HPLC purity is 99.78%, alcoholysis impurities are not detected, and base toxic impurities are not increased. Its powder diffraction data are as figure 2 shown.
[0050] That 1 The H-NMR data are as follows:
[0051] 1 H NMR (400MHz, d 6 -DMSO) δ9.00(d, J=6.5Hz, 1H), 8.72(s, 1H), 8.36(d, J=9.1Hz, 1H), 8.09(s, 1H), 7.95(d, J=24.3 Hz,2H),7....
Embodiment 2
[0056] Embodiment 2: Preparation of lenvatinib mesylate crystal form X
[0057] Disperse 10.0g of lenvatinib in 30ml of purified water and 30ml of acetonitrile, and stir at 30°C to form a suspension; then add the mixed solution of 2.7g of methanesulfonic acid, 10ml of acetic acid and 30ml of acetonitrile dropwise into the suspension; After the dropwise addition was completed and the suspension was dissolved, 3% seed crystal X was added, then 50ml of acetonitrile was added dropwise to the suspension, after the dropwise addition was completed, stirring was continued at 25°C for 12h, and the solid powder was obtained by suction filtration and drying to obtain 8.53g of solid powder. 85.3%. The HPLC purity is 99.77%, alcoholysis impurities are not detected, and base toxic impurities are not increased. Its powder diffraction data are basically consistent with the data of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com