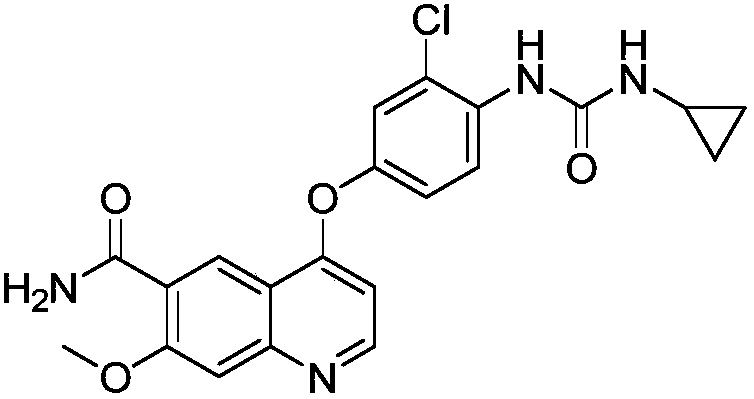
Synthesis method of lenvatinib and new intermediate
A synthetic method, the technology of lenvatinib, applied in the direction of organic chemistry, can solve the problems of separation and purification of intermediates, and achieve the effect of simple and easy operation and cheap and easy-to-obtain starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
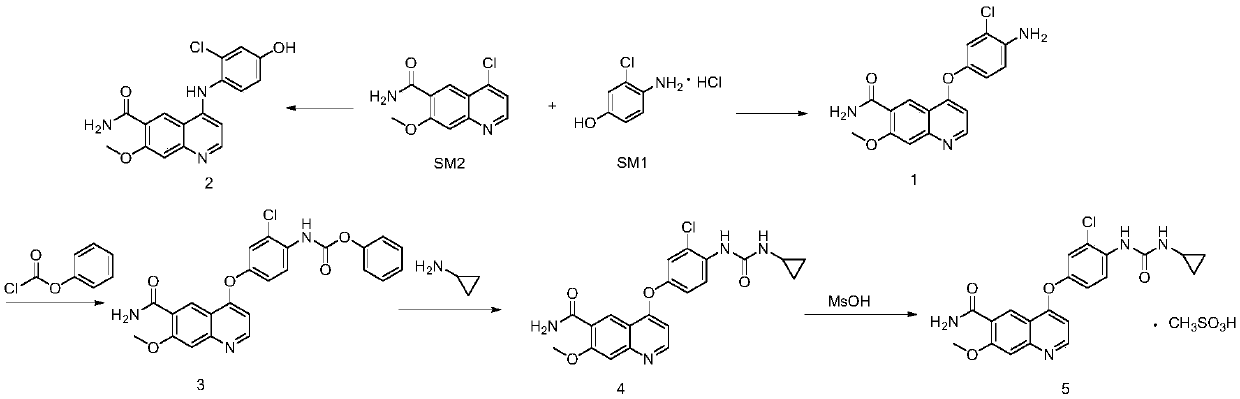
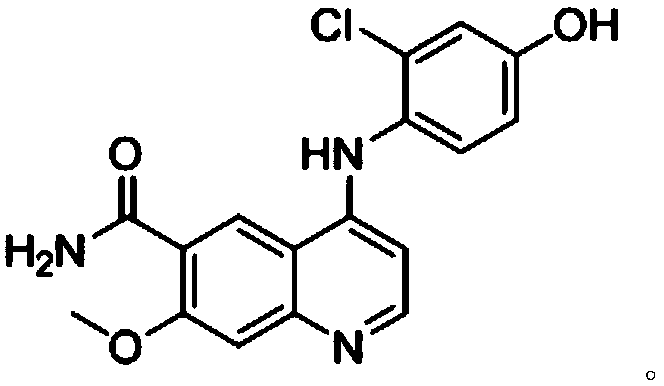
[0050] Preparation of compound 1:
[0051]
[0052] Weigh compound SM2 (2.00g, 8.45mmol), SM1 (2.13g, 11.83mmol) into a 100mL three-neck flask, add 18mL DMSO, and stir well. Weigh potassium hydroxide (1.19g, 21.13mmol) into a small beaker, add 2.4mL of water, stir to dissolve, and then add to the above-mentioned 100mL reaction flask. React at 80°C. After 24 hours of reaction, add a mixed solution of acetone and water until a large amount of brown solid precipitates, then filter with suction, and weigh the obtained solid after drying in a blast drying oven to obtain 2.75 g of crude product , the resulting crude product was recrystallized in DMSO to give a red solid. After drying in a blast drying oven, the compound 1 was obtained by weighing: 2.20 g, with a yield of 76%.
[0053] Preparation of compound 2:
[0054]
[0055]Weigh compound SM2 (30.00g, 126.76mmol), SM1 (27.36g, 151.97mmol) and cesium carbonate (83.34g, 255.94mmol) in a 500mL three-necked flask, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com