Crystal form of lenvatinib mesylate and preparation method thereof
A technology of nimethanesulfonate and crystal form, which is applied in the fields of organic chemistry, organic chemistry, antitumor drugs, etc. There are safety and other issues, and the preparation method is simple and effective, easy to scale up production, and the effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The invention provides a preparation method of the crystal form of lenvatinib mesylate, which comprises the following steps:
[0086] Step A: adding lenvatinib mesylate solid to a solvent to prepare a suspension; preferably, the solvent is selected from one or more of water, alcohols, ketones, alkanes, and ethers kind;
[0087] Step B: Add seed crystals to the suspension, add purified water dropwise, stir at a temperature of 20-30°C for 10-30 hours, then filter to obtain crystal slurry, and dry to constant weight to obtain the lenvatinib mesylate salt crystal form.
[0088] In the above preparation method of the present invention, the solvent may be a single solvent, or a mixture of two or more solvents, such as a mixture of water and alcohols, or a mixture of alcohols and ketones.
[0089] In a preferred embodiment, the solid form of lenvatinib mesylate is the solid form of lenvatinib mesylate, preferably an amorphous form.
[0090] In a preferred embodiment, the so...
Embodiment 1
[0123] Add 10g of lenvatinib mesylate solid to 400ml of methyl tert-butyl ether, stir at a speed of 300r / min, add 4ml of purified water dropwise, stir at 25°C for 12h, suction-filter the obtained crystal slurry, and store at a temperature of 45°C 1. Drying for 15 hours under a vacuum degree of -0.1Mpa, namely the crystalline form of derenvatinib mesylate, the purity is 99.4%, and the crystallization process yield is 91.0%.
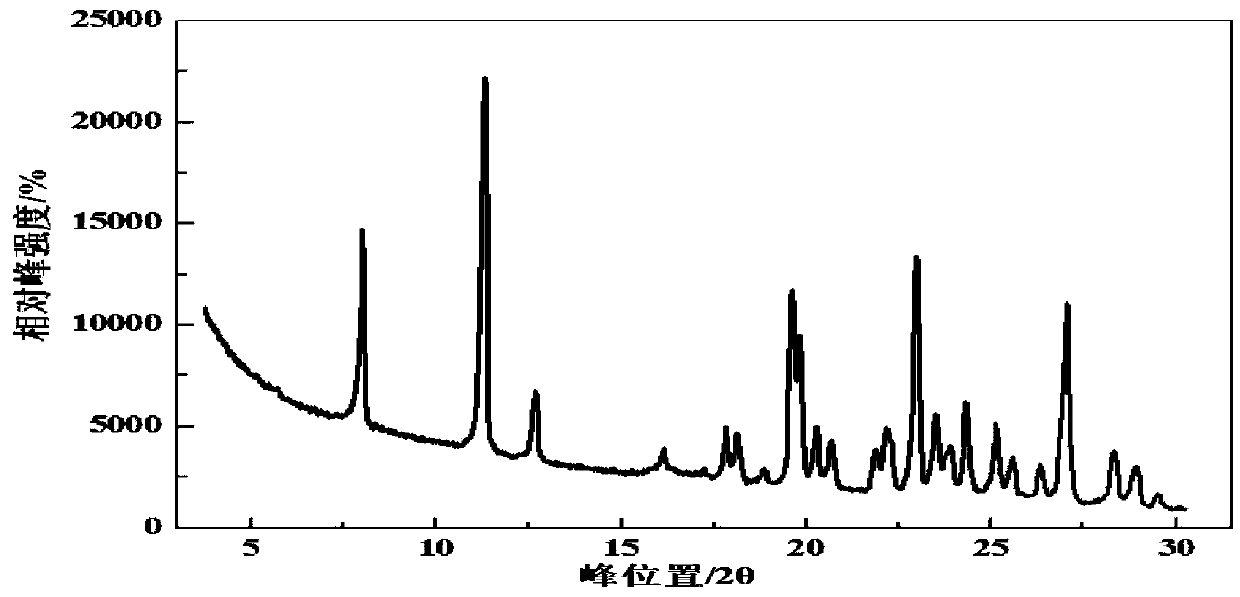
[0124] Carry out XRPD analysis to the obtained lenvatinib mesylate crystal form, its X-ray powder diffraction is in diffraction angle 2θ=5.6,8.0,11.2,12.6,16.1,17.2,17.8,18.1,18.9,19.6, There are characteristic peaks at 19.8, 20.2, 20.7, 21.8, 22.1, 22.2, 22.9, 23.5, 23.8, 24.3, 25.1, 25.6, 26.3, 27.0, 28.3, 28.8, 29.4, 30.0, 30.8, and 31.6 degrees.
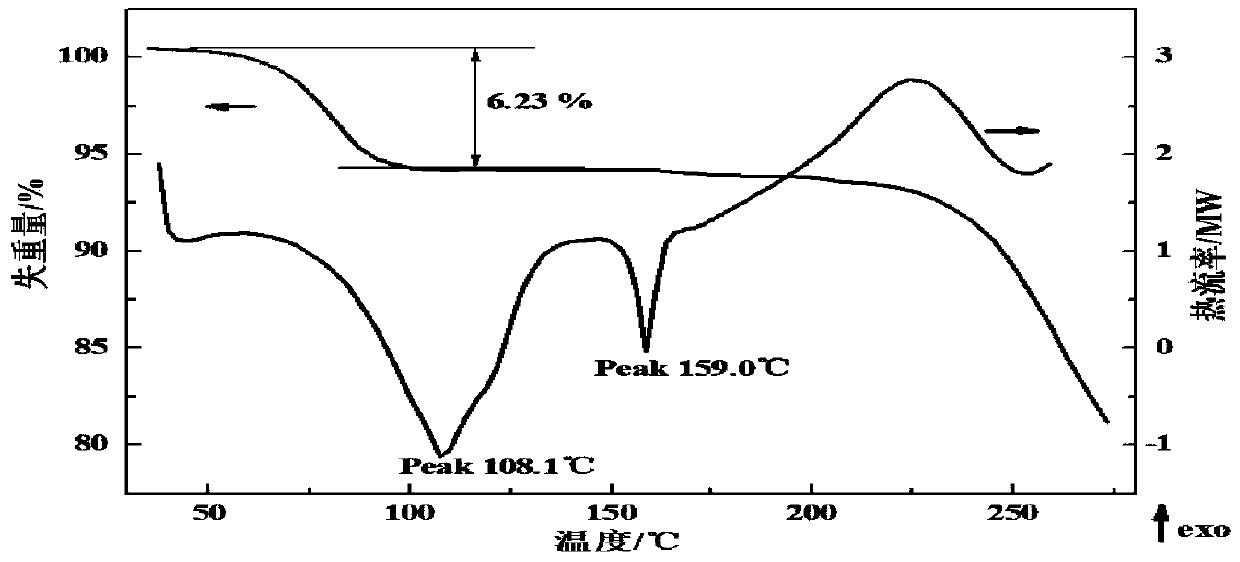
[0125] DSC analysis was performed on the obtained crystal form of lenvatinib mesylate, and it was analyzed that there were strong endothermic peaks at 108.1°C and 159.0°C.
[0126] The obtained crystal form ...
Embodiment 2
[0129] Add 10 g of lenvatinib mesylate solid to 400 ml of methyl tert-butyl ether, stir at a speed of 300 r / min, add 0.1 g of seed crystals, add dropwise 4 ml of purified water, stir at 20°C for 10 h, and filter the obtained crystals with suction. The slurry was dried at a temperature of 45° C. and a vacuum of -0.1 Mpa for 10 hours to obtain the crystalline form of derenvatinib mesylate with a purity of 99.6% and a crystallization yield of 90.0%.
[0130]Carry out XRPD analysis to the crystalline form of the obtained lenvatinib mesylate, the obtained XRPD spectrum is as follows figure 1 The relevant data are shown in Table 1.
[0131] Carry out DSC analysis to the crystal form of the obtained lenvatinib mesylate, such as figure 2 As shown, it has strong endothermic peaks at 108.1°C and 159.0°C.
[0132] Carry out TGA analysis to the crystal form of the obtained lenvatinib mesylate, such as figure 2 Shown, its water loss weight is 6.23%. Moisture determination by Karl Fis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com